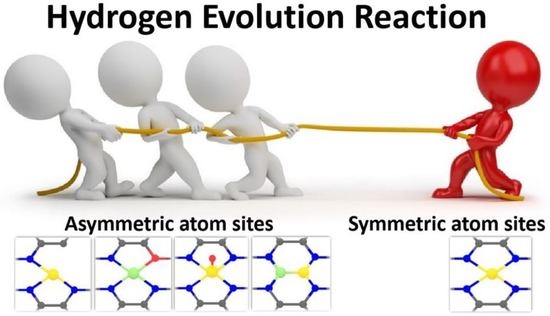
A Mini Review: Recent Advances in Asymmetrically Coordinated Atom Sites for High-Efficiency Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. The Mechanistic Principles of HER
3. Asymmetric Atom Sites for HER
3.1. Low−Coordination Structure
3.2. Lateral/Axial Heteroatom Coordination Structure
3.3. Dual−Metal Coordination Structure
3.4. Asymmetric Atom Sites of Organic/Metal−based Supports for HER
4. Summary and Outlook
Funding
Conflicts of Interest
References
- Wang, L.; Wang, D.; Li, Y. Single-atom catalysis for carbon neutrality. Carbon Energy 2022, 4, 1021–1079. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Vidal-Lopez, A.; Solà, M.; Poater, A. 2D Carbon Nitride as Support of Single Cu, Ag, and Au Atoms for Carbon Dioxide Reduction Reaction. Phys. Chem. Chem. Phys. 2023. [Google Scholar] [CrossRef]
- Gao, S.; Chen, S.; Liu, Q.; Zhang, S.; Qi, G.; Luo, J.; Liu, X. Bifunctional BiPd Alloy Particles Anchored on Carbon Matrix for Reversible Zn-CO2 Battery. ACS Appl. Nano Mater. 2022, 5, 12387–12394. [Google Scholar] [CrossRef]
- Musah, J.-D.; Ilyas, A.M.; Venkatesh, S.; Mensah, S.; Kwofie, S.; Roy, V.A.L.; Wu, C.-M.L. Isovalent substitution in metal chalcogenide materials for improving thermoelectric power generation-A critical review. Nano Res. Energy 2022, 1, 9120034. [Google Scholar] [CrossRef]
- Huhta, K. The contribution of energy law to the energy transition and energy research. Glob. Environ. Chang. 2022, 73, 102454. [Google Scholar] [CrossRef]
- Qi, D.; Lv, F.; Wei, T.; Jin, M.; Meng, G.; Zhang, S.; Liu, Q.; Liu, W.; Ma, D.; Hamdy, M.S.; et al. High-efficiency electrocatalytic NO reduction to NH3 by nanoporous VN. Nano Res. Energy 2022, 1, e9120022. [Google Scholar] [CrossRef]
- Payandeh, S.; Strauss, F.; Mazilkin, A.; Kondrakov, A.; Brezesinski, T. Tailoring the LiNbO3 coating of Ni-rich cathode materials for stable and high-performance all-solid-state batteries. Nano Res. Energy 2022, 1, e9120016. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Fan, M.; Yang, J.; Xiao, J.; Wang, Y. Future energy infrastructure, energy platform and energy storage. Nano Energy 2022, 104, 107915. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Zuo, Z. Advanced electrochemical energy storage and conversion on graphdiyne interface. Nano Res. Energy 2022, 1, 9120036. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Luo, Y.; Liu, Q.; Luo, J.; Chu, P.; Liu, X. Preparation of high entropy alloys and application to catalytical water electrolysis. APL Mater. 2022, 10, 070701. [Google Scholar] [CrossRef]
- Wang, W.; Hong, X.; Yao, Q.; Lu, Z.-H. Bimetallic Ni-Pt nanoparticles immobilized on mesoporous N-doped carbon as a highly efficient catalyst for complete hydrogen evolution from hydrazine borane. J. Mater. Chem. A 2020, 8, 13694–13701. [Google Scholar] [CrossRef]
- Wang, D.; Yang, P.; Liu, L.; Wang, W.; Chen, Z. Atomically dispersed metal-nitrogen-carbon electrocatalysts for oxygen reduction reaction: From synthesis strategies to activity engineering. Mater. Today Energy 2022, 26, 101017. [Google Scholar] [CrossRef]
- Wyvratt, B.M.; Gaudet, J.R.; Pardue, D.B.; Marton, A.; Rudić, S.; Mader, E.A.; Cundari, T.R.; Mayer, J.M.; Thompson, L.T. Reactivity of Hydrogen on and in Nanostructured Molybdenum Nitride: Crotonaldehyde Hydrogenation. ACS Catal. 2016, 6, 5797–5806. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Y.; Yu, X.; Qin, Y.; Meng, T.; Hu, X. The pursuit of commercial silicon-based microparticle anodes for advanced lithium-ion batteries: A review. Nano Res. Energy 2022, 1, 9120037. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Ge, R.; Zhang, J.; Cairney, J.M.; Li, Y.; Zhu, M.; Li, S.; Li, W. The effect of coordination environment on the activity and selectivity of single-atom catalysts. Coord. Chem. Rev. 2022, 461, 214493. [Google Scholar] [CrossRef]
- Su, H.; Soldatov, M.A.; Roldugin, V.; Liu, Q. Platinum single-atom catalyst with self-adjustable valence state for large-current-density acidic water oxidation. eScience 2022, 2, 102–109. [Google Scholar] [CrossRef]
- Liu, W.; Feng, J.; Wei, T.; Liu, Q.; Zhang, S.; Luo, Y.; Luo, J.; Liu, X. Active-site and interface engineering of cathode materials for aqueous Zn-gas batteries. Nano Res. 2023, 16, 2325–2346. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, X.; Zhang, L.; Lang, R.; Qiao, B. Single-atom catalysis: Bridging the homo- and heterogeneous catalysis. Chin. J. Catal. 2018, 39, 893–898. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, T.; Qiu, Y.; Zhang, S.; Liu, Q.; Hu, G.; Luo, J.; Liu, X. Recent Progress in Metal Phosphorous Chalcogenides: Potential High-Performance Electrocatalysts. Small 2023. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wei, J.; Qiu, X.; Jiao, N. Homogeneous Oxygenase Catalysis. Chem. Rev. 2018, 118, 4912–4945. [Google Scholar] [CrossRef] [PubMed]
- Cornils, B. Catalysis. Concepts and Green Applications. By Gadi Rothenberg. Angew. Chem. Int. Ed. 2008, 47, 5500–5501. [Google Scholar] [CrossRef]
- Sun, Z.; Wen, X.; Wang, L.; Ji, D.; Qin, X.; Yu, J.; Ramakrishna, S. Emerging design principles, materials, and applications for moisture-enabled electric generation. eScience 2022, 2, 32–46. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Y.; Zhang, S.S.; Liu, Q.; Luo, J.; Liu, X.J. Nitrogen-incorporated iron phosphosulfide nanosheets as efficient bifunctional electrocatalysts for energy-saving hydrogen evolution. Ionics 2022, 28, 3927–3934. [Google Scholar] [CrossRef]
- Rozzi, E.; Minuto, F.D.; Lanzini, A.; Leone, P. Green Synthetic Fuels: Renewable Routes for the Conversion of Non-Fossil Feedstocks into Gaseous Fuels and Their End Uses. Energies 2020, 13, 420. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Luo, Y.; Chu, P.; Liu, Q.; Liu, X.; Zhang, S.; Luo, J.; Wang, X.; Hu, G. Recent advances in non-noble metal-based bifunctional electrocatalysts for overall seawater splitting. J. Alloy. Compd. 2022, 922, 166113. [Google Scholar] [CrossRef]
- Heppe, N.; Gallenkamp, C.; Paul, S.; Segura-Salas, N.; von Rhein, N.; Kaiser, B.; Jaegermann, W.; Jafari, A.; Sergueev, I.; Krewald, V.; et al. Substituent Effects in Iron Porphyrin Catalysts for the Hydrogen Evolution Reaction. Chem. Eur. J. 2023, 29, e202202465. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Ariga, K.; Yamauchi, Y. Single-Atom Catalysts. Small 2021, 17, 2101584. [Google Scholar] [CrossRef] [PubMed]
- Agwa, A.M.; El-Fergany, A.A.; Sarhan, G.M. Steady-State Modeling of Fuel Cells Based on Atom Search Optimizer. Energies 2019, 12, 1884. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Yang, H.; Zhang, S.; Liu, Q.; Cao, H.; Luo, J.; Liu, X. Advances in the Electrocatalytic Hydrogen Evolution Reaction by Metal Nanoclusters-based Materials. Small 2022, 18, 2204524. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cui, M.; Yin, Z.; Xiong, J.; Mi, L.; Li, Y. Single-Atom and Dual-Atom Electrocatalysts Derived from Metal Organic Frameworks: Current Progress and Perspectives. ChemSusChem 2021, 14, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ou, P.; Liu, W.; Wang, X.; Wang, H.-T.; Zhang, R.; Pao, C.-W.; Liu, X.; Pong, W.-F.; Song, J.; et al. Design of Ru-Ni diatomic sites for efficient alkaline hydrogen oxidation. Sci. Adv. 2022, 8, eabm3779. [Google Scholar] [CrossRef]
- Cao, H.; Wei, T.; Liu, Q.; Zhang, S.; Qin, Y.; Wang, H.; Luo, J.; Liu, X. Hollow Carbon Cages Derived from Polyoxometalate-Encapsuled Metal-Organic Frameworks for Energy-Saving Hydrogen Production. ChemCatChem 2023. [Google Scholar] [CrossRef]
- Grishakov, K.S.; Katin, K.P.; Kochaev, A.I.; Kaya, S.; Gimaldinova, M.A.; Maslov, M.M. Ab initio Study of Hydrogen Adsorption on Metal-Decorated Borophene-Graphene Bilayer. Energies 2021, 14, 2473. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Jia, B.; Wang, X.; Jiang, S.; Ma, T. Isolating Single and Few Atoms for Enhanced Catalysis. Adv. Mater. 2022, 34, e2201796. [Google Scholar] [CrossRef]
- Jin, M.; Liu, S.; Meng, G.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. Low-Coordinated Mo Clusters for High-Efficiency Electrocatalytic Hydrogen Peroxide Production. Adv. Mater. Interfaces 2023, 10, 2201144. [Google Scholar] [CrossRef]
- Hou, X.; Ding, J.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Asymmetric Coordination Environment Engineering of Atomic Catalysts for CO2 Reduction. Nanomaterials 2023, 13, 309. [Google Scholar] [CrossRef]
- Dobrota, A.S.; Skorodumova, N.V.; Mentus, S.V.; Pašti, I.A. Surface pourbaix plots of M@N4-graphene single-atom electrocatalysts from density functional theory thermodynamic modeling. Electrochim. Acta 2022, 412, 140155. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Kao, C.-W.; Luo, T.; Chen, K.; Fu, J.; Ma, C.; Li, H.; Li, M.; Chan, T.-S.; et al. Unveiling the Proton-Feeding Effect in Sulfur-Doped Fe-N-C Single-Atom Catalyst for Enhanced CO2 Electroreduction. Angew. Chem. Int. Ed. 2022, 61, e202206233. [Google Scholar]
- Antil, B.; Kumar, L.; Ranjan, R.; Shenoy, S.; Tarafder, K.; Gopinath, C.S.; Deka, S. One-Dimensional Multichannel g-C3N4.7 Nanostructure Realizing an Efficient Photocatalytic Hydrogen Evolution Reaction and Its Theoretical Investigations. ACS Appl. Energy Mater. 2021, 4, 3118–3129. [Google Scholar] [CrossRef]
- Cui, J.; Liu, X.; Wei, Y.; Shen, X. A Synergistic effect on the atomic cluster M4 supported on MN4-graphene (M = Fe, Ni) for the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2022, 24, 11704–11712. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Liu, W.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. A dual-functional Bi-doped Co3O4 nanosheet array towards high efficiency 5-hydroxymethylfurfural oxidation and hydrogen production. Chem. Commun. 2023, 59, 442–445. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wu, J.; Zhao, X.; Xiong, Y.; Luo, F.; Lei, Y. Asymmetric atomic sites make different: Recent progress in electrocatalytic CO2 reduction. Nano Energy 2022, 103, 107815. [Google Scholar] [CrossRef]
- Xu, W.; Tang, H.; Gu, H.; Xi, H.; Wu, P.; Liang, B.; Liu, Q.; Chen, W. Research progress of asymmetrically coordinated single-atom catalysts for electrocatalytic reactions. J. Mater. Chem. A 2022, 10, 14732–14746. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Sun, J.; Zhang, S.; Liu, X.; Luo, J. Integration of partially phosphatized bimetal centers into trifunctional catalyst for high-performance hydrogen production and flexible Zn-air battery. Sci. China Mater. 2022, 65, 1176–1186. [Google Scholar] [CrossRef]
- Liu, H.; Fu, J.; Li, H.; Sun, J.; Liu, X.; Qiu, Y.; Peng, X.; Liu, Y.; Bao, H.; Zhuo, L.; et al. Single palladium site in ordered porous heteroatom-doped carbon for high-performance alkaline hydrogen oxidation. Appl. Catal. B Environ. 2022, 306, 121029. [Google Scholar] [CrossRef]
- Cometto, C.; Ugolotti, A.; Grazietti, E.; Moretto, A.; Bottaro, G.; Armelao, L.; Di Valentin, C.; Calvillo, L.; Granozzi, G. Copper single-atoms embedded in 2D graphitic carbon nitride for the CO2 reduction. npj 2D Mater. Appl. 2021, 5, 63. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Zocche, N.; Lorenzi, R.; Ferrara, C.; Poli, F.; Soavi, F.; Santoro, C. Valorization of the inedible pistachio shells into nanoscale transition metal and nitrogen codoped carbon-based electrocatalysts for hydrogen evolution reaction and oxygen reduction reaction. Mater. Renew. Sustain. Energy 2022, 11, 131–141. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Zhang, M.; Gou, W.; Zhang, S.; Chen, Z.; Qu, Y.; Ma, Y. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution. Nat. Commun. 2021, 12, 3502. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, L.; Hou, J.; Liu, S.; Qin, Y.; Liu, Q.; Cai, X.; Sun, Z.; Yang, M.; Luo, J.; et al. Cu2O-Derived PtCu Nanoalloy toward Energy-Efficient Hydrogen Production via Hydrazine Electrolysis under Large Current Density. ACS Appl. Energy Mater. 2022, 5, 9487–9494. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Single-Atom Transition Metal Photocatalysts for Hydrogen Evolution Reactions. Catalysts 2022, 12, 1304. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, L.; Zhang, Q.; Teng, Z.; Sun, H.; Su, C. TiO2-supported Single-atom Catalysts: Synthesis, Structure, and Application. Chem. Res. Chin. Univ. 2022, 38, 1123–1138. [Google Scholar] [CrossRef]
- Hruby, V.; Zaoralova, D.; Medved, M.; Bakandritsos, A.; Zboril, R.; Otyepka, M. Emerging graphene derivatives as active 2D coordination platforms for single-atom catalysts. Nanoscale 2022, 14, 13490–13499. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Z.; Qiu, B.; Xing, M.; Zhang, J. Emerging Cocatalysts on g-C3N4 for Photocatalytic Hydrogen Evolution. Small 2021, 17, 2101070. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Hao, X.-F.; Jiang, Z.; Sun, X.-P.; Xu, D.; Wang, J.; Zhong, H.-X.; Meng, F.-L.; Zhang, X.-B. C and N Hybrid Coordination Derived Co-C-N Complex as a Highly Efficient Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 15070–15073. [Google Scholar] [CrossRef] [PubMed]
- Di Liberto, G.; Cipriano, L.A.; Pacchioni, G. Role of Dihydride and Dihydrogen Complexes in Hydrogen Evolution Reaction on Single-Atom Catalysts. J. Am. Chem. Soc. 2021, 143, 20431–20441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.-A.; Huang, Y.; Mu, H.; Gu, X.; Li, F.; Wang, Z.; Li, J. Impacts of Metal-Support Interaction on Hydrogen Evolution Reaction of Cobalt-Nitride-Carbide Catalyst. Front. Chem. 2022, 9, 828964. [Google Scholar] [CrossRef]
- Cepitis, R.; Kongi, N.; Grozovski, V.; Ivaništšev, V.; Lust, E. Multifunctional Electrocatalysis on Single-Site Metal Catalysts: A Computational Perspective. Catalysts 2021, 11, 1165. [Google Scholar] [CrossRef]
- Zang, W.; Sun, T.; Yang, T.; Xi, S.; Waqar, M.; Kou, Z.; Lyu, Z.; Feng, Y.P.; Wang, J.; Pennycook, S.J. Efficient Hydrogen Evolution of Oxidized Ni-N3 Defective Sites for Alkaline Freshwater and Seawater Electrolysis. Adv. Mater. 2021, 33, 2003846. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, L.; Han, C.; Zong, H.; Yang, G.; Lin, S.; Kumar, A.; Jadhav, A.R.; Tran, N.Q.; Hwang, Y.; et al. Identifying the Activity Origin of a Cobalt Single-Atom Catalyst for Hydrogen Evolution Using Supervised Learning. Adv. Funct. Mater. 2021, 31, 2100547. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Y.; Gao, G.; Yan, X.; Chen, N.; Chen, J.; Soo, M.T.; Wood, B.; Yang, D.; Du, A.; et al. Graphene Defects Trap Atomic Ni Species for Hydrogen and Oxygen Evolution Reactions. Chem 2018, 4, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.-J.; Ito, Y.; Cong, W.; Tan, Y.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. Nanoporous Graphene with Single-Atom Nickel Dopants: An Efficient and Stable Catalyst for Electrochemical Hydrogen Production. Angew. Chem. Int. Ed. 2015, 54, 14031–14035. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhu, X.; Liu, X.; Gu, J.; Liu, W.; Wang, D.; Zhang, W.; Lin, Y.; Lu, J.; Wei, S.; et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Huang, K.; Wang, D.; Zhang, R.; Ge, B.; Ma, J.; Wen, B.; Zhang, S.; Li, Q.; Lei, M.; et al. Iced photochemical reduction to synthesize atomically dispersed metals by suppressing nanocrystal growth. Nat. Commun. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Lu, B.; Chen, L.; Wang, N.; Lu, J.E.; Ping, Y.; Chen, S. Hydrogen evolution reaction catalyzed by ruthenium ion-complexed graphitic carbon nitride nanosheets. J. Mater. Chem. A 2017, 5, 18261–18269. [Google Scholar] [CrossRef]
- Yin, X.-P.; Wang, H.-J.; Tang, S.-F.; Lu, X.-L.; Shu, M.; Si, R.; Lu, T.-B. Engineering the Coordination Environment of Single-Atom Platinum Anchored on Graphdiyne for Optimizing Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 9382–9386. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Guo, L.; Wu, F.; Peng, Y.; Lu, J.E.; Smart, T.J.; Wang, N.; Finfrock, Y.Z.; Morris, D.; Zhang, P.; et al. Ruthenium atomically dispersed in carbon outperforms platinum toward hydrogen evolution in alkaline media. Nat. Commun. 2019, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Pei, J.; He, C.-T.; Wan, J.; Ren, H.; Wang, Y.; Dong, J.; Wu, K.; Cheong, W.-C.; Mao, J.; et al. Single Tungsten Atoms Supported on MOF-Derived N-Doped Carbon for Robust Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1800396. [Google Scholar] [CrossRef]
- Lai, W.-H.; Zhang, L.-F.; Hua, W.-B.; Indris, S.; Yan, Z.-C.; Hu, Z.; Zhang, B.; Liu, Y.; Wang, L.; Liu, M.; et al. General π-Electron-Assisted Strategy for Ir, Pt, Ru, Pd, Fe, Ni Single-Atom Electrocatalysts with Bifunctional Active Sites for Highly Efficient Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 11868–11873. [Google Scholar] [CrossRef]
- Jin, H.; Sultan, S.; Ha, M.; Tiwari, J.N.; Kim, M.G.; Kim, K.S. Simple and Scalable Mechanochemical Synthesis of Noble Metal Catalysts with Single Atoms toward Highly Efficient Hydrogen Evolution. Adv. Funct. Mater. 2020, 30, 2000531. [Google Scholar] [CrossRef]
- Chen, W.; Pei, J.; He, C.-T.; Wan, J.; Ren, H.; Zhu, Y.; Wang, Y.; Dong, J.; Tian, S.; Cheong, W.-C.; et al. Rational Design of Single Molybdenum Atoms Anchored on N-Doped Carbon for Effective Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2017, 56, 16086–16090. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhao, Z.; Shang, H.; Peng, B.; Chen, W.; Pei, J.; Zheng, L.; Dong, J.; Cao, R.; Sarangi, R.; et al. In Situ Phosphatizing of Triphenylphosphine Encapsulated within Metal-Organic Frameworks to Design Atomic Co1-P1N3 Interfacial Structure for Promoting Catalytic Performance. J. Am. Chem. Soc. 2020, 142, 8431–8439. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, D.; Huang, K.; Dong, J.; Liao, J.; Dai, S.; Tang, X.; Yan, M.; Gong, H.; Liu, J.; et al. Iodine-Doping-Induced Electronic Structure Tuning of Atomic Cobalt for Enhanced Hydrogen Evolution Electrocatalysis. ACS Nano 2021, 15, 18125–18134. [Google Scholar] [CrossRef]
- Li, T.; Lu, T.; Li, X.; Xu, L.; Zhang, Y.; Tian, Z.; Yang, J.; Pang, H.; Tang, Y.; Xue, J. Atomically Dispersed Mo Sites Anchored on Multichannel Carbon Nanofibers toward Superior Electrocatalytic Hydrogen Evolution. ACS Nano 2021, 15, 20032–20041. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Cheng, Z.; Tan, Y.; Liu, S.; Shen, Z. Doping sp-hybridized B atoms in graphyne supported single cobalt atoms for hydrogen evolution electrocatalysis. Int. J. Hydrogen Energy 2019, 44, 27421–27428. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, E.; Chen, W.; Segre, C.U.; Zhou, J.; Lin, Y.-C.; Zhu, C.; Ma, R.; Liu, P.; Chu, S.; et al. Dual-Metal Interbonding as the Chemical Facilitator for Single-Atom Dispersions. Adv. Mater. 2020, 32, 2003484. [Google Scholar] [CrossRef]
- Zhang, L.; Si, R.; Liu, H.; Chen, N.; Wang, Q.; Adair, K.; Wang, Z.; Chen, J.; Song, Z.; Li, J.; et al. Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction. Nat. Commun. 2019, 10, 4936. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Song, E.; Wang, M.; Chen, W.; Huang, F.; Feng, Z.; Liu, J.; Wang, J. Partial-Single-Atom, Partial-Nanoparticle Composites Enhance Water Dissociation for Hydrogen Evolution. Adv. Sci. 2021, 8, 2001881. [Google Scholar] [CrossRef]
- Zeng, X.; Shui, J.; Liu, X.; Liu, Q.; Li, Y.; Shang, J.; Zheng, L.; Yu, R. Single-Atom to Single-Atom Grafting of Pt1 onto Fe-N4 Center: Pt1@Fe-N-C Multifunctional Electrocatalyst with Significantly Enhanced Properties. Adv. Energy Mater. 2018, 8, 1701345. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, Y.; Li, H.; Zhang, Z.; Mu, Y.; Do, D.; Zhou, B.; Dong, J.; Yan, W.; Qin, Y.; et al. O-coordinated W-Mo dual-atom catalyst for pH-universal electrocatalytic hydrogen evolution. Sci. Adv. 2020, 6, eaba6586. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhao, Y.; Bai, Y.; Li, F.; Zhang, Y.; Chen, Y. Conductive Metal-Organic Frameworks with Extra Metallic Sites as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Adv. Sci. 2020, 7, 2000012. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-P.; Zhou, W.; Zhao, J.; Dong, W.-W.; Lan, Y.-Q.; Li, D.-S.; Sun, C.; Bu, X. Surfactant-Assisted Phase-Selective Synthesis of New Cobalt MOFs and Their Efficient Electrocatalytic Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2017, 56, 13001–13005. [Google Scholar] [CrossRef]
- Lin, H.-W.; Senthil Raja, D.; Chuah, X.-F.; Hsieh, C.-T.; Chen, Y.-A.; Lu, S.-Y. Bi-metallic MOFs possessing hierarchical synergistic effects as high performance electrocatalysts for overall water splitting at high current densities. Appl. Catal. B Environ. 2019, 258, 118023. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Gómez-García, C.J.; Ma, H.; Zhang, C.; Pang, H.; Li, B. Two Novel Polyoxometalate-Encapsulated Metal-Organic Nanotube Frameworks as Stable and Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 31498–31504. [Google Scholar] [CrossRef]
- Chao, T.; Luo, X.; Chen, W.; Jiang, B.; Ge, J.; Lin, Y.; Wu, G.; Wang, X.; Hu, Y.; Zhuang, Z.; et al. Atomically Dispersed Copper-Platinum Dual Sites Alloyed with Palladium Nanorings Catalyze the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2017, 56, 16047–16051. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Luo, Q.; Liu, W.; Lin, Y.; Liu, X.; Cao, Y.; Zhang, W.; Wu, Y.; Yang, J.; Yao, T.; et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat. Catal. 2019, 2, 134–141. [Google Scholar] [CrossRef]
- Liang, H.-W.; Brüller, S.; Dong, R.; Zhang, J.; Feng, X.; Müllen, K. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, H.; Dong, J.; Wan, C.; Zhao, Z.; Xu, X.; Lin, Z.; Wang, Y.; Liu, H.; Zang, K.; Luo, J.; et al. Microwave-Assisted Rapid Synthesis of Graphene-Supported Single Atomic Metals. Adv. Mater. 2018, 30, 1802146. [Google Scholar] [CrossRef]
- Zhang, H.; An, P.; Zhou, W.; Guan, B.Y.; Zhang, P.; Dong, J.; Lou, X.W. Dynamic traction of lattice-confined platinum atoms into mesoporous carbon matrix for hydrogen evolution reaction. Sci. Adv. 2018, 4, eaao6657. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Luo, F.; Zhang, Q.; Zhang, P.; Xu, T.; Wang, Q.; He, D.; Guo, L.; Zhang, Y.; He, C.; et al. Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction. Energy Environ. Sci. 2019, 12, 1000–1007. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Song, Y.; Wang, F. Photochemical Solid-Phase Synthesis of Platinum Single Atoms on Nitrogen-Doped Carbon with High Loading as Bifunctional Catalysts for Hydrogen Evolution and Oxygen Reduction Reactions. ACS Catal. 2018, 8, 8450–8458. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Liu, Z.; Liu, S.; Chen, Y.; Li, M.; Zhang, H.; Wu, Q.; Guo, J.; Feng, X.; et al. Synergetic Function of the Single-Atom Ru-N4 Site and Ru Nanoparticles for Hydrogen Production in a Wide pH Range and Seawater Electrolysis. ACS Appl. Mater. Interfaces 2022, 14, 15250–15258. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Liu, X.; Liu, W.; Li, Z.; Dong, J.; Chen, W.; Yan, W.; Yao, T.; Duan, X.; et al. Efficient and Robust Hydrogen Evolution: Phosphorus Nitride Imide Nanotubes as Supports for Anchoring Single Ruthenium Sites. Angew. Chem. Int. Ed. 2018, 57, 9495–9500. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wang, Y.; Hou, Y.; Liu, P.; Yang, J.; Zhang, T.; Zhuang, X.; Chen, M.; Yang, B.; Lei, L.; et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni-Nx Species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics. Energy Environ. Sci. 2019, 12, 149–156. [Google Scholar] [CrossRef]
- Hou, J.; Peng, X.; Sun, J.; Zhang, S.; Liu, Q.; Wang, X.; Luo, J.; Liu, X. Accelerating hydrazine-assisted hydrogen production kinetics with Mn dopant modulated CoS2 nanowire arrays. Inorg. Chem. Front. 2022, 9, 3047–3058. [Google Scholar] [CrossRef]
- Hu, G.; Wu, Z.; Jiang, D.-e. Stronger-than-Pt hydrogen adsorption in a Au22 nanocluster for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 7532–7537. [Google Scholar] [CrossRef]
- Hossain, M.D.; Liu, Z.; Zhuang, M.; Yan, X.; Xu, G.-L.; Gadre, C.A.; Tyagi, A.; Abidi, I.H.; Sun, C.-J.; Wong, H.; et al. Rational Design of Graphene-Supported Single Atom Catalysts for Hydrogen Evolution Reaction. Adv. Energy Mater. 2019, 9, 1803689. [Google Scholar] [CrossRef]
- Shen, H.; Wei, T.; Liu, Q.; Zhang, S.; Luo, J.; Liu, X. Heterogeneous Ni-MoN nanosheet-assembled microspheres for urea-assisted hydrogen production. J. Colloid Interface Sci. 2023, 634, 730–736. [Google Scholar] [CrossRef]
- Cui, X.; Gao, L.; Lu, C.-H.; Ma, R.; Yang, Y.; Lin, Z. Rational coordination regulation in carbon-based single-metal-atom catalysts for electrocatalytic oxygen reduction reaction. Nano Convergence 2022, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Sato, K.; Nagata, T.; Hagiwara, H.; Watanabe, M.; Kim, N.; Shiota, Y.; Koinuma, M.; Takenaka, S.; Sakai, T.; et al. A Cocatalyst that Stabilizes a Hydride Intermediate during Photocatalytic Hydrogen Evolution over a Rhodium-Doped TiO2 Nanosheet. Angew. Chem. Int. Ed. 2018, 57, 9073–9077. [Google Scholar] [CrossRef]
- Gao, S.; Wei, T.; Sun, J.; Liu, Q.; Ma, D.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Atomically Dispersed Metal-Based Catalysts for Zn-CO2 Batteries. Small Struct. 2022, 3, 2200086. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Y.; Tian, W.; Zhang, H.; Li, J.; Wang, S.; Wang, Y. Non-Metal Single-Phosphorus-Atom Catalysis of Hydrogen Evolution. Angew. Chem. Int. Ed. 2020, 59, 23791–23799. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Kang, L.; Gaocan, Q.; Shusheng, Z.; Qian, L.; Yang, L.; Jun, L.; Xijun, L. High Entropy Alloys in Water Electrolysis: Recent advances, Fundamentals, and Challenges. Sci. China Mater. 2022. [Google Scholar] [CrossRef]
- Huan, T.N.; Ranjbar, N.; Rousse, G.; Sougrati, M.; Zitolo, A.; Mougel, V.; Jaouen, F.; Fontecave, M. Electrochemical Reduction of CO2 Catalyzed by Fe-N-C Materials: A Structure–Selectivity Study. ACS Catal. 2017, 7, 1520–1525. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Wang, T.; Jin, M.; Zhang, S.; Liu, Q.; Hu, G.; Yang, H.; Luo, J.; Liu, X. Bifunctional Nb-N-C atomic catalyst for aqueous Zn-air battery driving CO2 electrolysis. Sci. China Mater. 2022. [Google Scholar] [CrossRef]
- Maher, A.G.; Passard, G.; Dogutan, D.K.; Halbach, R.L.; Anderson, B.L.; Gagliardi, C.J.; Taniguchi, M.; Lindsey, J.S.; Nocera, D.G. Hydrogen Evolution Catalysis by a Sparsely Substituted Cobalt Chlorin. ACS Catal. 2017, 7, 3597–3606. [Google Scholar] [CrossRef]
- Meng, G.; Cao, H.; Wei, T.; Liu, Q.; Fu, J.; Zhang, S.; Luo, J.; Liu, X. Highly dispersed Ru clusters toward an efficient and durable hydrogen oxidation reaction. Chem. Commun. 2022, 58, 11839–11842. [Google Scholar] [CrossRef]
- Hu, B.; Huang, A.; Zhang, X.; Chen, Z.; Tu, R.; Zhu, W.; Zhuang, Z.; Chen, C.; Peng, Q.; Li, Y. Atomic Co/Ni dual sites with N/P-coordination as bifunctional oxygen electrocatalyst for rechargeable zinc-air batteries. Nano Res. 2021, 14, 3482–3488. [Google Scholar] [CrossRef]
- Zhu, P.; Xiong, X.; Wang, D. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction. Nano Res. 2022, 15, 5792–5815. [Google Scholar] [CrossRef]
- Ge, M.; Mengmeng, J.; Tianran, W.; Qian, L.; Shusheng, Z.; Xianyun, P.; Jun, L.; Xijun, L. MoC nanocrystals confined in N-doped carbon nanosheets toward highly selective electrocatalytic nitric oxide reduction to ammonia. Nano Res. 2022, 15, 8890–8896. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Ye, Y.; Lee, S.; Park, J.; Lee, H.; Lee, J.; Han, J.W. Rational Design of TiC-Supported Single-Atom Electrocatalysts for Hydrogen Evolution and Selective Oxygen Reduction Reactions. ACS Energy Lett. 2019, 4, 126–132. [Google Scholar] [CrossRef]
- Meng, G.; Wei, T.; Liu, W.; Li, W.; Zhang, S.; Liu, W.; Liu, Q.; Bao, H.; Luo, J.; Liu, X. NiFe layered double hydroxide nanosheet array for high-efficiency electrocatalytic reduction of nitric oxide to ammonia. Chem. Commun. 2022, 58, 8097–8100. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, L.; Wulan, B.; Tan, D.; Chen, Q.; Ma, J.; Zhang, J. Atomic Bridging Structure of Nickel–Nitrogen–Carbon for Highly Efficient Electrocatalytic Reduction of CO2. Angew. Chem. Int. Ed. 2022, 61, e202113918. [Google Scholar]
- Kwon, I.S.; Kwak, I.H.; Debela, T.T.; Abbas, H.G.; Park, Y.C.; Ahn, J.-p.; Park, J.; Kang, H.S. Se-Rich MoSe2 Nanosheets and Their Superior Electrocatalytic Performance for Hydrogen Evolution Reaction. ACS Nano 2020, 14, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, L.-H.; Du, J.; Wang, H.; Guo, J.; Zhan, J.; Li, F.; Yu, F. A Tandem Strategy for Enhancing Electrochemical CO2 Reduction Activity of Single-Atom Cu-S1N3 Catalysts via Integration with Cu Nanoclusters. Angew. Chem. Int. Ed. 2021, 60, 24022–24027. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Li, W.-H.; Zhang, Z.; Zhao, X.-C.; Cao, W.; Huang, Q.-S. Ni optimizes Ir reaction pathway through IrNi alloy synergistic effect to improve overall water splitting efficiency. Int. J. Hydrogen Energy 2023, 48, 8440–8449. [Google Scholar] [CrossRef]
- Yang, M.; Liu, S.; Sun, J.; Jin, M.; Fu, R.; Zhang, S.; Li, H.; Sun, Z.; Luo, J.; Liu, X. Highly dispersed Bi clusters for efficient rechargeable Zn−CO2 batteries. Appl. Catal. B: Environ. 2022, 307, 121145. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, F.; Jin, M.; Zhao, D.; Fan, X.; Li, Z.; Luo, Y.; Zheng, D.; Li, T.; Wang, Y.; et al. V-doped TiO2 nanobelt array for high-efficiency electrocatalytic nitrite reduction to ammonia. Mater. Today Phys. 2023, 30, 100944. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Y.; Hu, J.; Zhang, H.; Luo, P.; Sun, F.; Ma, X.; Huang, Z.; Li, J.; Guo, Z.; et al. Surface-Electron Coupling for Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2019, 58, 17709–17717. [Google Scholar] [CrossRef]
- Tkachenko, P.; Volchek, V.; Kurenkova, A.; Gerasimov, E.; Popovetskiy, P.; Asanov, I.; Yushina, I.; Kozlova, E.; Vasilchenko, D. Photocatalytic H2 generation from ethanol and glucose aqueous solutions by PtOx/TiO2 composites. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Wang, T.; Gao, S.; Wei, T.; Qin, Y.; Zhang, S.; Ding, J.; Liu, Q.; Luo, J.; Liu, X. Co nanoparticles confined in mesoporous Mo/N co-doped polyhedral carbon frameworks towards high-efficiency oxygen reduction. Chem. Eur. J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Li, R.; Zang, H.-Y.; Tan, H.-Q.; Kang, Z.-H.; Wang, Y.-H.; Li, Y.-G. Advanced hydrogen evolution electrocatalysts promising sustainable hydrogen and chlor-alkali co-production. Energy Environ. Sci. 2021, 14, 6191–6210. [Google Scholar] [CrossRef]
- Kwak, I.H.; Abbas, H.G.; Kwon, I.S.; Park, Y.C.; Seo, J.; Cho, M.K.; Ahn, J.-P.; Seo, H.W.; Park, J.; Kang, H.S. Intercalation of cobaltocene into WS2 nanosheets for enhanced catalytic hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 8101–8106. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Bao, H.; Wang, X.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. Ionic Liquid-Assisted Electrocatalytic NO Reduction to NH3 by P-Doped MoS2. ChemCatChem 2023, 15, e202201411. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, X.; Lin, Z.; Zeng, X.; Zhu, Y.; Wang, Y.; Gong, M.; Tang, Y.; Fu, G. Ethanol-Induced Hydrogen Insertion in Ultrafine IrPdH Boosts pH-Universal Hydrogen Evolution. Small 2022, 18, 2204063. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Qin, Y.; Yang, H.; Zhang, S.; Liu, X.; Luo, J. Hollow CoFe-layered double hydroxide polyhedrons for highly efficient CO2 electrolysis. Sci. China Mater. 2022, 65, 536–542. [Google Scholar] [CrossRef]
- Li, B.; Yu, S.; Zhou, M.; Chen, C.; Lai, C.; Zhang, M.; Lin, H. Graphynes: Ideal supports of single atoms for electrochemical energy conversion. J. Mater. Chem. A 2022, 10, 3905–3932. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, H.; Zhang, S.; Meng, G.; Liu, Q.; Sun, Z.; Luo, J.; Liu, X. Polycrystalline SnSx nanofilm enables CO2 electroreduction to formate with high current density. Chem. Commun. 2022, 58, 7654–7657. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhu, H.; Zhuang, Z.; Lu, S.; Duan, F.; Du, M. Single-atom catalysts for electrochemical clean energy conversion: Recent progress and perspectives. Sustain. Energy Fuels 2020, 4, 996–1011. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Yang, H.; Gao, S.; Liu, Y.; Zhang, S.; Chen, Y.; Liu, X.; Luo, J. Nitrogen-Doped Carbon Polyhedrons Confined Fe-P Nanocrystals as High-Efficiency Bifunctional Catalysts for Aqueous Zn-CO2 Batteries. Small 2022, 18, 2104965. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, L.; Tu, Y.; Zhang, L.; Zhang, W. Emerging ruthenium single-atom catalysts for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2022, 10, 15370–15389. [Google Scholar] [CrossRef]
- Steier, L.; Holliday, S. A bright outlook on organic photoelectrochemical cells for water splitting. J. Mater. Chem. A 2018, 6, 21809–21826. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Wang, Z.; Zhang, S.; Ding, J.; Luo, J.; Liu, X. Cathode materials for halide-based aqueous redox flow batteries: Recent progress and future perspectives. Nanoscale 2023, 15, 4250–4260. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, S.; Yu, X.; Li, K.; Ni, X.; Ye, L. Nitrogen-doped carbon spheres with precisely-constructed pyridinic-N active sites for efficient oxygen reduction. Appl. Surf. Sci. 2022, 598, 153786. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Singh, A.N.; Sultan, S.; Kim, K.S. Recent Advancement of p- and d-Block Elements, Single Atoms, and Graphene-Based Photoelectrochemical Electrodes for Water Splitting. Adv. Energy Mater. 2020, 10, 2000280. [Google Scholar] [CrossRef]
- Hoa, V.H.; Tran, D.T.; Prabhakaran, S.; Kim, D.H.; Hameed, N.; Wang, H.; Kim, N.H.; Lee, J.H. Ruthenium single atoms implanted continuous MoS2-Mo2C heterostructure for high-performance and stable water splitting. Nano Energy 2021, 88, 106277. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, S.; Liu, Q.; Qiu, Y.; Luo, J.; Liu, X. Oxygen Vacancy-Rich Amorphous Copper Oxide Enables Highly Selective Electroreduction of Carbon Dioxide to Ethylene. Acta Phys. Chim. Sin. 2023, 39, 202207026. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Zhang, W.; Chen, Y.; Peng, X.; Wang, X.; Zhao, W.; Qin, C.; Liu, Q.; Liu, X.; et al. Single-Atomic Co-B2N2 Sites Anchored on Carbon Nanotube Arrays Promote Lithium Polysulfide Conversion in Lithium Sulfur Batteries. Carbon Energy 2022. [Google Scholar] [CrossRef]
- Liu, W.; Feng, J.; Yin, R.; Ni, Y.; Zheng, D.; Que, W.; Niu, X.; Dai, X.; Shi, W.; Wu, F.; et al. Tailoring oxygenated groups of monolithic cobalt-nitrogen-carbon frameworks for highly efficient hydrogen peroxide production in acidic media. Chem. Eng. J. 2022, 430, 132990. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, D.; Yin, R.; Niu, X.; Xu, X.; Meng, S.; Ma, S.; Shi, W.; Wu, F.; Liu, W.; et al. A Wide-Temperature Adaptive Aqueous Zinc-Air Battery-Based on Cu-Co Dual Metal-Nitrogen-Carbon/Nanoparticle Electrocatalysts. Small Struct. 2023, 2200340. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, X.; Wei, T.; Liu, Q.; Luo, J.; Liu, X. Single atomic cerium sites anchored on nitrogen-doped hollow carbon spheres for highly selective electroreduction of nitric oxide to ammonia. J. Colloid Interface Sci. 2023, 638, 650–657. [Google Scholar] [CrossRef]
- Jin, J.; Chen, F.; Feng, Y.; Zhou, J.; Lei, W.; Gao, F. Co-Ni-Mo phosphides hierarchical nanoarrays as bifunctional electrocatalysts for excellent overall water splitting. Fuel 2023, 332, 126131. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Luo, L.; Du, Y.; Cheng, X.; Wu, Q. Nitrogen-doped Fe2O3/NiTe2 as an excellent bifunctional electrocatalyst for overall water splitting. J. Colloid Interface Sci. 2023, 639, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Han, X.; Li, Y.; Han, A.; Liu, W.; Xu, H.; Liu, J. Hollow Mesoporous Metal-Organic Frameworks with Enhanced Diffusion for Highly Efficient Catalysis. ACS Catal. 2020, 10, 5973–5978. [Google Scholar] [CrossRef]
- Lu, M.; Li, L.; Chen, D.; Li, J.; Klyui, N.I.; Han, W. MOF-derived nitrogen-doped CoO@CoP arrays as bifunctional electrocatalysts for efficient overall water splitting. Electrochim. Acta 2020, 330, 135210. [Google Scholar] [CrossRef]
- Yang, H.; Ma, D.; Li, Y.; Zhao, Q.; Pan, F.; Zheng, S.; Lou, Z. Mo Doped Ru-based Cluster to Promote Alkaline Hydrogen Evolution with Ultra-Low Ru Loading. Chin. J. Struct. Chem. 2023, 100031. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Z.; Lin, Z.; Shen, S.; Zhong, W. Fabricating Ru single atoms and clusters on CoP for boosted hydrogen evolution reaction. Chin. J. Struct. Chem. 2023, 100035. [Google Scholar] [CrossRef]
- Liu, W.; Que, W.; Yin, R.; Dai, J.; Zheng, D.; Feng, J.; Xu, X.; Wu, F.; Shi, W.; Liu, X.; et al. Ferrum-molybdenum dual incorporated cobalt oxides as efficient bifunctional anti-corrosion electrocatalyst for seawater splitting. Appl. Catal. B Environ. 2023, 328, 122488. [Google Scholar] [CrossRef]






| Catalyst | Active Site | Electrolyte | Current Density (mA cm−2), Overpotential (mV) | Stability | Ref. |
|---|---|---|---|---|---|
| E−Co SAs | CoN2 | 1 M KOH | 10, ~59 | 500 mA cm−2, 200 h | [59] |
| CoN4 | CoN4 | 1 M KOH | 10, ~150 | − | |
| Co1/PCN | CoN4 | 1 M KOH | 10, 89 | 10 mA cm−2, 24 h | [85] |
| CoNx/C | CoN4 | 1 M KOH | 10, 170 | − | [86] |
| CoN4 | 0.5 M H2SO4 | 10, 133 | LSVs, 5000 cycles | ||
| Co−NG−MW | CoN4 | 0.5 M H2SO4 | 10, 127 | LSVs, 1000 cycles | [87] |
| Co−SA−without P | CoN4 | 0.5 M H2SO4 | 10, 148 | − | [71] |
| Co−SA/P−in situ | CoP1N3 | 0.5 M H2SO4 | 10, 98 | LSVs, 1000 cycles | [71] |
| Pt@PCM | PtN4 | 0.5 M H2SO4 | 10, 105 | ~22.5 mA cm−2, 5 h | [88] |
| Pt SACs/AG | PtN4 | 0.5 M H2SO4 | 10, 12 | LSVs, 2000 cycles | [89] |
| Pt1/NPC | PtN4 | 0.5 M H2SO4 | 10, 25 | LSVs, 3000 cycles | [90] |
| Pt1/MC | PtC3 | 0.5 M H2SO4 | 10, ~27 | LSVs, 1000 cycles | [63] |
| Pt@NC−B | PtN2C2 | 0.5 M H2SO4 | 10, 39 | 10 mA cm−2, 40 h | [69] |
| Pt1/OLC | PtO2C1 | 0.5 M H2SO4 | 10, ~38 | 10 mA cm−2, 100 h | [91] |
| Ru−NC−700 | RuC2N2 | 1 M KOH | 10, 12 | LSVs, 10,000 cycles | [66] |
| Ru1/N−C | RuN4 | 1 M KOH | 10, 173 | − | [92] |
| RuN4 | 0.5 M H2SO4 | 10, 282 | − | ||
| Ru SAs@PN | RuN4 | 0.5 M H2SO4 | 10, 24 | LSVs, 5000 cycles | [93] |
| Ni−SA/NC | NiN3 | 1 M KOH | 10, 102 | LSVs, 5000 cycles | [58] |
| Ni−N−C | NiN4 | 1 M KOH | 10, 307 | − | [94] |
| Nisub/G | NiC3 | 0.5 M H2SO4 | 10, ~50 | ~9 mA cm−2, 120 h | [61] |
| W−SA | WN1C3 | 0.1 M KOH | 10, 85 | LSVs, 10,000 cycles | [67] |
| Mo1N1C2 | MoN1C2 | 0.1 M KOH | 10, 132 | LSVs, 1000 cycles | [70] |
| Mo@NMCNFs | MoN1C2−O1 | 0.5 M H2SO4 | 10, 66 | LSVs, 3000 cycles | [73] |
| FR−NCS | Rh−FeN4 | 0.5 M H2SO4 | 10, 22 | LSVs, 1000 cycles | [75] |
| Pt1@Fe−N−C | Pt−O2−FeN4 | 0.5 M H2SO4 | 10, 60 | 5 mA cm−2, 5.5 h | [78] |
| W1Mo1−NG | W−O−Mo−O−C | 0.5 M H2SO4 | 10, 24 | 10 mA cm−2, 100,000 s | [79] |
| 1 M KOH | 10, 67 | 10 mA cm−2, 100,000 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. A Mini Review: Recent Advances in Asymmetrically Coordinated Atom Sites for High-Efficiency Hydrogen Evolution Reaction. Energies 2023, 16, 2664. https://doi.org/10.3390/en16062664
Ding J, Liu W, Zhang S, Luo J, Liu X. A Mini Review: Recent Advances in Asymmetrically Coordinated Atom Sites for High-Efficiency Hydrogen Evolution Reaction. Energies. 2023; 16(6):2664. https://doi.org/10.3390/en16062664
Chicago/Turabian StyleDing, Junyang, Wenxian Liu, Shusheng Zhang, Jun Luo, and Xijun Liu. 2023. "A Mini Review: Recent Advances in Asymmetrically Coordinated Atom Sites for High-Efficiency Hydrogen Evolution Reaction" Energies 16, no. 6: 2664. https://doi.org/10.3390/en16062664