A Novel Approach Using High Charging Voltage for the Restoration of Discarded Lead Acid Batteries
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Experimental Setup
2.3. Procedure
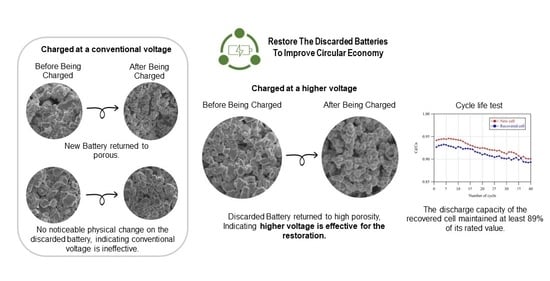
3. Results and Discussion
3.1. Case One: New and Discarded Cells Charged at 2.4 V
3.1.1. Positive Plates
3.1.2. Negative Plates
3.2. Case Two: Discarded Batteries
3.2.1. Charging Results for Failed Batteries
3.2.2. Charging Results for Recovered Batteries
3.3. Case Three: Discarded Cells
3.3.1. Charging and Discharging Results for Recovered Cells
3.3.2. Surface Morphology Study of the Recovered Cells
3.3.3. Cycle Life Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Byrne, R.H.; Nguyen, T.A.; Copp, D.A.; Chalamala, B.R.; Gyuk, I. Energy Management and Optimization Methods for Grid Energy Storage Systems. IEEE Access 2018, 6, 13231–13260. [Google Scholar] [CrossRef]
- Gong, H.; Rallabandi, V.; McIntyre, M.L.; Hossain, E.; Ionel, D.M. Peak Reduction and Long Term Load Forecasting for Large Residential Communities Including Smart Homes with Energy Storage. IEEE Access 2021, 9, 19345–19355. [Google Scholar] [CrossRef]
- Hau, L.C.; Lim, Y.S.; Chua, K.H. Active Control Strategy of Energy Storage System for Reducing Maximum Demand Charges under Limited Storage Capacity. J. Energy Eng. 2017, 143, 04017010. [Google Scholar] [CrossRef]
- Chua, K.H.; Lim, Y.S.; Morris, S. Peak Reduction for Commercial Buildings Using Energy Storage. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kunming, China, 22–25 September 2017; Volume 93, p. 012008. [Google Scholar] [CrossRef]
- Marańda, W. Capacity degradation of lead-acid batteries under variable-depth cycling operation in photovoltaic system. In Proceedings of the 2015 22nd International Conference Mixed Design of Integrated Circuits & Systems, Torun, Poland, 25–27 June 2015; pp. 552–555. [Google Scholar] [CrossRef]
- Yahmadi, R.; Brik, K.; Ammar, F.B. Degradation analysis of the lead acid battery plates in the manufacturing process. In Proceedings of the 2015 IEEE 12th International Multi-Conference on Systems, Signals & Devices, Mahdia, Tunisia, 16–19 March 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Delgado-Sanchez, J.M.; Lillo-Bravo, I. Influence of Degradation Processes in Lead-acid Batteries on the Technoeconomic Analysis of Photovoltaic Systems. Energies 2020, 13, 4075. [Google Scholar] [CrossRef]
- Yahmadi, R.; Brik, K.; Ammar, F.B. Failures Analysis and Improvement Lifetime of Lead Acid Battery in Different Applications. In Proceedings of the 3rd International Conference on Automation, Control, Engineering and Computer Science, Hammamet, Tunisia, 20–22 March 2016; pp. 148–154. [Google Scholar]
- Ruetschi, P. Aging Mechanisms and Service Life of Lead–Acid Batteries. J. Power Sources 2004, 127, 33–44. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B. Lead Batteries for Utility Energy Storage: A Review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries; McGraw Hill, Ed.; Elsevier Science: New York, NY, USA, 2002. [Google Scholar]
- Kirchev, A.; Delaille, A.; Perrin, M.; Lemaire, E.; Mattera, F. Studies of the Pulse Charge of Lead-acid Batteries for PV Applications. Part II. Impedance of the Positive Plate Revisited. J. Power Sources 2007, 170, 495–512. [Google Scholar] [CrossRef]
- Hu, J.; Guo, Y. Effects of Electrolyte Stratification on Performances of AGM Valve-regulated Lead-acid Batteries. Electrochim. Acta 2007, 52, 6734–6740. [Google Scholar] [CrossRef]
- Catherino, H.A.; Feres, F.F.; Trinidad, F. Sulfation in Lead-acid Batteries. J. Power Sources 2004, 129, 113–120. [Google Scholar] [CrossRef]
- Franke, M.; Kowal, J. Empirical Sulfation Model for Valve-regulated Lead-acid Batteries Under Cycling Operation. J. Power Sources 2018, 380, 76–82. [Google Scholar] [CrossRef]
- Pavlov, D.; Milusheva, Y.; Vassilev, S.; Shibahara, T.; Tozuka, M. Benzyl Benzoate as an Inhibitor of the Sulfation of Negative Electrodes in Lead Acid Batteries. J. Energy Storage 2018, 17, 336–344. [Google Scholar] [CrossRef]
- Ghavami, R.K.; Kameli, F.; Shirojan, A.; Azizi, A. Effects of Surfactants on Sulfation of Negative Active Material in Lead Acid Battery Under PSOC Condition. J. Energy Storage 2016, 7, 121–130. [Google Scholar] [CrossRef]
- Moseley, P.T.; Rand, D.A.J.; Garche, J. Lead-Acid Batteries for Future Automobiles; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–618. [Google Scholar] [CrossRef]
- Taguchi, M.; Sugita, H. Analysis for Electrolytic Oxidation and Reduction of PbSO4/Pb Electrode by Electrochemical QCM Technique. J. Power Sources 2002, 109, 294–300. [Google Scholar] [CrossRef]
- Zeng, Y.; Hu, J.; Ye, W.; Zhao, W.; Zhou, G. Investigation of Lead Dendrite Growth in the Formation of Valve-regulated Lead-acid Batteries for Electric Bicycle Applications. J. Power Sources 2015, 286, 182–192. [Google Scholar] [CrossRef]
- Lakshmi, C.S. Structure and Properties of Lead–calcium–tin Alloys for Battery Grids. J. Power Sources 1998, 73, 23–29. [Google Scholar] [CrossRef]
- Peixoto, L.C.; Osório, W.R.; Garcia, A. Microstructure and Electrochemical Corrosion Behavior of a Pb–1 wt % Sn Alloy for Lead-Acid Battery Components. J. Power Sources 2009, 192, 724–729. [Google Scholar] [CrossRef]
- Rand, D.A.J.; Boden, D.P.; Lakshmi, C.S.; Nelson, R.F.; Prengaman, R.D. Manufacturing and Operational Issues with Lead-acid Batteries. J. Power Sources 2002, 107, 280–300. [Google Scholar] [CrossRef]
- Slavkov, D.; Haran, B.S.; Popov, B.N.; Fleming, F. Effect of Sn and Ca Doping on the Corrosion of Pb Anodes in Lead Acid Batteries. J. Power Sources 2002, 112, 199–208. [Google Scholar] [CrossRef]
- Thomas, U.B.; Forster, F.T.; Haring, H.E. Corrosion and Growth of Lead-calcium Alloy Storage Battery Grids asAFunction of Calcium Content. Trans. Electrochem. Soc. 1947, 92, 313–325. [Google Scholar] [CrossRef]
- Pour-ali, S.; Mosallami, M.; Davoodi, A. Electrochemical Corrosion Behavior of Pb-Ca-Sn-Sm Grid Alloy in H2SO4 Solution. J. Alloys Compd. 2015, 652, 172–178. [Google Scholar] [CrossRef]
- Moseley, P.T.; Garche, J. Electrochemical Energy Storage for Renewable Sources and Grid Balancing. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Newnes: Oxford, UK, 2014; pp. 1–473. [Google Scholar] [CrossRef]
- Rahmanifar, M.S. Electrochimica Acta Enhancing the Cycle Life of Lead-Acid Batteries by Modifying Negative Grid Surface. Electrochim. Acta 2017, 235, 10–18. [Google Scholar] [CrossRef]
- Boden, D.P. Improved Oxides for Production of Lead/acid Battery Plates. J. Power Sources 1998, 73, 56–59. [Google Scholar] [CrossRef]
- Schilling, S. Ensuring Lead-acid Battery Performance with Pulse Technology. In Proceedings of the Fourteenth Annual Battery Conference on Applications and Advances, Long Beach, CA, USA, 12–15 January 1999; pp. 247–252. [Google Scholar] [CrossRef]
- Hsieh, H.I.; Shih, S.F.; Hsieh, J.H.; Wang, C.H. Photovoltaic High-frequency Pulse Charger for Lead-acid Battery Under Maximum Power Point Tracking. Int. J. Photoenergy 2013, 2013, 687693. [Google Scholar] [CrossRef]
- Sun, R.L.; Hu, P.Q.; Wang, R.; Qi, L.Y. A New Method for Charging and Repairing Lead-acid Batteries. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Harbin, China, 29 November–1 December 2020; Volume 461, p. 012031. [Google Scholar] [CrossRef]
- Kirchev, A.; Delaille, A.; Karoui, F.; Perrin, M.; Lemaire, E.; Mattera, F. Studies of the Pulse Charge of Lead-acid Batteries for PV Applications. Part III. Electrolyte Concentration Effects on the Electrochemical Performance of the Positive Plate. J. Power Sources 2008, 179, 808–818. [Google Scholar] [CrossRef]
- Kirchev, A.; Mattera, F.; Lemaire, E.; Dong, K. Studies of the Pulse Charge of Lead-acid Batteries for Photovoltaic Applications. Part IV. Pulse Charge of the Negative Plate. J. Power Sources 2009, 191, 82–90. [Google Scholar] [CrossRef]
- Horkos, P.G.; Yammine, E.; Karami, N. Review on Different Charging Techniques of Lead-acid Batteries. In Proceedings of the 2015 3rd International Conference on Technological Advances in Electrical, Electronics and Computer Engineering (TAEECE), Beirut, Lebanon, 29 April 2015–1 May 2015; Volume 2015, pp. 27–32. [Google Scholar] [CrossRef]
- Karami, H.; Asadi, R. Recovery of discarded sulfated lead-acid batteries. J. Power Sources 2009, 191, 165–175. [Google Scholar] [CrossRef]
- Khalil, R.A. A study of the effect of inverse charging on the service life of lead acid battery. Bull. Electrochem. 2000, 16, 472–475. [Google Scholar]
- Spanos, C.; Berlinger, S.A.; Jayan, A.; West, A.C. Inverse Charging Techniques for Sulfation Reversal in Flooded Lead-Acid Batteries. J. Electrochem. Soc. 2016, 163, A1612–A1618. [Google Scholar] [CrossRef]
- Vinal, G.W.; Craig, D.N. Resistivity of Sulfuric-acid Solutions and Its Relation to Viscosity and Temperature. J. Res. Natl. Bur. Stand. 1934, 13, 689. [Google Scholar] [CrossRef]
- Svoboda, V. Batteries|Fast Charging. Encycl. Electrochem. Power Sources 2009, 424–442. [Google Scholar] [CrossRef]
- He, Q.; Zha, Y.; Sun, Q.; Pan, Z.; Liu, T. Capacity Fast Prediction and Residual Useful Life Estimation of Valve Regulated Lead Acid Battery. Math. Probl. Eng. 2017, 2017, 7835049. [Google Scholar] [CrossRef]
- Ioannou, S.; Dalamagkidis, K.; Stefanakos, E.K.; Valavanis, K.P.; Wiley, P.H. Runtime, Capacity and Discharge Current Relationship for Lead Acid and Lithium Batteries. In Proceedings of the 2016 24th Mediterranean Conference on Control and Automation (MED), Athens, Greece, 21–24 June 2016; Volume 2016, pp. 46–53. [Google Scholar] [CrossRef]
- Cugnet, M.G.; Dubarry, M.; Liaw, B.Y. Peukert’s Law of a Lead-Acid Battery Simulated by a Mathematical Model. ECS Trans. 2019, 25, 223–233. [Google Scholar] [CrossRef]













| Charging Voltage (V) | Set Current | |
|---|---|---|
| Batteries | Cells | |
| 14.8 | 2.46 | 0.077 C |
| 15.2 | 2.53 | 0.077 C |
| 15.6 | 2.60 | 0.077 C |
| 16.0 | 2.67 | 0.077 C |
| Description | Components (% wt) | ||
|---|---|---|---|
| Pb | S | O | |
| New positive plate before charging | 75.34 | 11.89 | 12.77 |
| New positive plate after charging | 80.55 | 06.21 | 13.24 |
| Discarded positive plate before charging | 71.40 | 14.10 | 14.50 |
| Discarded positive plate after charging | 71.03 | 12.84 | 16.13 |
| Description | Components (% wt) | ||
|---|---|---|---|
| Pb | S | O | |
| New negative plate before charging | 79.92 | 07.44 | 12.64 |
| New negative plate after charging | 86.47 | 03.66 | 09.87 |
| Discarded negative plate before charging | 77.76 | 08.89 | 13.35 |
| Discarded negative plate after charging | 78.20 | 08.71 | 13.09 |
| Scenario | Charging Voltage (V) | Max. Recorded Current C (A) | Results | ||
|---|---|---|---|---|---|
| Battery Temp. at 20th h (°C) | Charging Response | Open Circuit Voltage (V) | |||
| 1 | 14.8 | 0.003 C (0.02 A) | 28.0 | No acceptance of charge | <12 |
| 1 | 15.2 | 0.013 C (0.09 A) | 28.3 | No acceptance of charge | <12 |
| 2 | 15.6 | 0.067 C (0.48 A) | 34.2 | Voltage-on-charge was stable | <12 |
| 3 | 15.6 | 0.077 C (0.55 A) | 47.0 | Voltage fluctuated between 12.6 and 15.3 V | >12 |
| 2 | 16.0 | 0.071 C (0.51 A) | 34.9 | Voltage-on-charge was stable | <12 |
| 3 | 16.0 | 0.077 C (0.55 A) | 47.0 | Voltage fluctuated between 12.6 and 15.1 V | >12 |
| Charging Voltage (V) | Max. Recorded Current C (A) | Results | ||
|---|---|---|---|---|
| Battery Temp. at 20th h (°C) | Charging Response | Open Circuit Voltage (V) | ||
| 15.6 | 0.077 C (0.55 A) | 32.3 | Voltage-on-charge achieved 15.6 V | >12 |
| 16.0 | 0.077 C (0.55 A) | 33.1 | Voltage-on-charge achieved 16.0 V | >12 |
| Battery No. | Charging Voltage (V) | Discharge Capacity (mAh) | Cf/Ci | % of Recovery | |
|---|---|---|---|---|---|
| Before (Ci) | After (Cf) | ||||
| 1 | 15.6 | 320 | 6440 | 20.1 | 85.0 |
| 2 | 15.6 | 401 | 6289 | 15.7 | 81.8 |
| 3 | 15.6 | 386 | 5856 | 15.2 | 76.2 |
| 4 | 15.6 | 343 | 5128 | 15.0 | 66.5 |
| 5 | 16.0 | 690 | 5709 | 8.3 | 69.7 |
| Charging Voltage (V) | Maximum Current (A) | Electrolyte (mL) | Results | ||
|---|---|---|---|---|---|
| Before Charge | After Charge | Charging Response | 5 h Discharge Capacity (mAh) | ||
| 2.46 | 0.01 | 160 | 160 | No acceptance of charge | -- |
| 2.53 | 0.03 | 160 | 160 | No acceptance of charge | 18 |
| 2.60 | 0.09 (0.077 C) | 160 | 160 | Fully charged | 1112 |
| 2.67 | 0.09 (0.077 C) | 160 | 156 | Fully charged | 1134 |
| Sample | Components (% wt) | ||
|---|---|---|---|
| Pb | S | O | |
| Positive plate before charging | 70.12 | 14.37 | 15.51 |
| Positive plate after charging | 71.10 | 11.07 | 17.83 |
| Negative plate before charging | 65.85 | 15.78 | 18.37 |
| Negative plate after charging | 83.67 | 06.25 | 10.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.H.; Wong, J.; Lim, Y.S. A Novel Approach Using High Charging Voltage for the Restoration of Discarded Lead Acid Batteries. Energies 2023, 16, 1598. https://doi.org/10.3390/en16041598
Lee CH, Wong J, Lim YS. A Novel Approach Using High Charging Voltage for the Restoration of Discarded Lead Acid Batteries. Energies. 2023; 16(4):1598. https://doi.org/10.3390/en16041598
Chicago/Turabian StyleLee, Chee Hiun, Jianhui Wong, and Yun Seng Lim. 2023. "A Novel Approach Using High Charging Voltage for the Restoration of Discarded Lead Acid Batteries" Energies 16, no. 4: 1598. https://doi.org/10.3390/en16041598