Cracking of Waste Engine Oil in the Presence of Fe3O4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. WEO Thermogravimetric Experiment
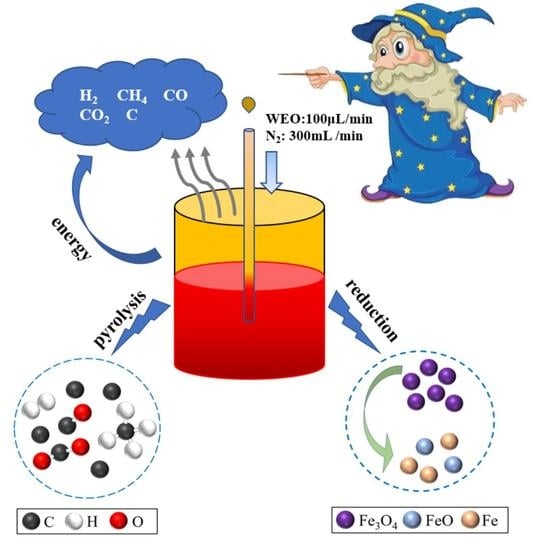
2.2.2. Thermal Cracking of WEO
2.2.3. Instruments
3. Results and Discussion
3.1. Thermal Cracking of WEO
3.1.1. Thermogravimetric Analysis of WEO Pyrolysis
3.1.2. FTIR Analysis
3.1.3. Infrared Spectroscopic Analysis of WEO Pyrolysis
3.2. Thermal Cracking Characteristics of WEO
3.2.1. Hydrogen
3.2.2. Methane
3.2.3. Carbon Monoxide and Carbon Dioxide
3.2.4. Carbon Deposition
3.2.5. Solid Product Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| The instantaneous total gas flow | |
| WEO | Waste engine oil |
| The instantaneous volume fraction of N2 | |
| The instantaneous volume fraction of gas species (i: H2, CH4, CO, CO2, CnHm) | |
| The total amount of each gas | |
| The volumefraction of each gas at time (i: H2, CH4, CO, CO2, CnHm) |
References
- Maceiras, R.; Alfonsín, V.; Morales, F.J. Recycling of waste engine oil for diesel production. Waste Manag. 2016, 60, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Boughton, B.; Horvath, A. Environmental assessment of used oil management methods. Environ. Sci. Technol. 2004, 38, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agamuthu, P.; Abioye, O.P.; Aziz, A.A. Phytoremediation of soil contaminated with used lubricating oil using jatropha curcas. J. Hazard. Mater. 2010, 179, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Nivedita, P.; Krushna, P.S.; Pravin, K.K. Characterization of waste engine oil derived pyrolytic char (WEOPC): SEM, EDX and FTIR analysis. Mater. Today Proc. 2021, 38, 2866–2870. [Google Scholar] [CrossRef]
- Hamawand, I.; Yusaf, T.; Rafat, S. Recycling of waste engine oils using a new washing agent. Energies 2013, 6, 1023–1049. [Google Scholar] [CrossRef]
- Vks, A.; Rk, B.; Aps, A. Laboratory investigation of bituminous concrete using reclaimed asphalt pavement and waste engine oil. Mater. Today Proc. 2022, 59, 1591–1598. [Google Scholar] [CrossRef]
- Chen, A.; Hu, Z.; Li, M.; Bai, T.; Xie, G.; Zhang, Y.; Li, Y.; Li, C. Investigation on the mechanism and performance of asphalt and its mixture regenerated by waste engine oil. Constr. Build. Mater. 2021, 313, 125411. [Google Scholar] [CrossRef]
- Al-Mutlaqa, S.; Mahal, E.; Yahya, R.; Mahal, A. Effect of chlorination on the assessment of waste engine oil modified asphalt binders. Pet. Sci. Technol. 2019, 37, 617–628. [Google Scholar] [CrossRef]
- Liu, S.; Meng, H.; Xu, Y.; Zhou, S. Evaluation of rheological characteristics of asphalt modified with waste engine oil (WEO). Pet. Sci. Technol. 2018, 36, 475–480. [Google Scholar] [CrossRef]
- Fernandes, S.R.M.; Silva, H.M.R.D.; Oliveira, J.R.M. Developing enhanced modified bitumens with waste engine oil products combined with polymers. Constr. Build. Mater. 2018, 160, 714–724. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, S.H. Pyrolysis kinetics of waste automobile lubricating oil. Fuel 2000, 79, 1943–1949. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Chase, H.A. Catalytic microwave pyrolysis of waste engine oil using metallic pyrolysis char. Appl. Catal. B Environ. 2015, 176, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.S.; Russell, A.D.; Chase, H.A. Microwave pyrolysis, a novel process for recycling waste automotive engine oil. Energy 2010, 35, 2985–2991. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Chase, H.A. Pyrolysis using microwave heating: A sustainable process for recycling used car engine oil. Ind. Eng. Chem. Res. 2010, 49, 10845–10851. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Moliner, R. Co-pyrolysis of a mineral waste oil/coal slurry in a continuous-mode fluidized bed reactor-ScienceDirect. J. Anal. Appl. Pyrolysis 2002, 65, 239–252. [Google Scholar] [CrossRef]
- Blanco, P.H.; Wu, C.; Onwudili, J.A.; Williams, P.T. Characterization and evaluation of Ni/SiO catalysts for hydrogen production and tar reduction from catalytic steam pyrolysis-reforming of refuse derived fuel. Appl. Catal. B Environ. 2013, 134, 238–250. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q.; Ma, D.; Takahashi, F.; Yoshikawa, K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl. Catal. B Environ. 2014, 152, 140–151. [Google Scholar] [CrossRef]
- Zouad, Y.; Tarabet, L.; Khiari, K.; Mahmoud, R. Effect of heating rate and additives (MgO and Al2O3 ) on a diesel like-fuel issued from waste engine oil pyrolysis. Pet. Sci. Technol. 2019, 37, 1184–1193. [Google Scholar] [CrossRef]
- Bhaskar, T.; Uddin, M.A.; Muto, A.; Sakata, Y.; Omura, Y.; Kimura, K.; Kawakami, Y. Recycling of waste lubricant oil into chemical feedstock or fuel oil over supported iron oxide catalysts. Fuel 2004, 83, 9–15. [Google Scholar] [CrossRef]
- Abdul-Raouf, M.E.; Maysour, N.E.; Abdul-Azim, A.; Amin, M.S. Thermochemical recycling of mixture of scrap tyres and waste lubricating oil into high caloric value products. Energy Convers. Manag. 2010, 51, 1304–1310. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Zhang, S.; Li, B.; Wang, H. Reduction of copper smelting slag using waste cooking oil. J. Clean. Prod. 2019, 236, 117668. [Google Scholar] [CrossRef]
- Zhou, L.; Zong, Z.; Tang, S.; Zong, Y.; Xie, R.; Ding, M.; Zhao, W.; Zhu, X.; Xia, Z.; Wu, L.; et al. FTIR and mass spectral analyses of an upgraded Bio-oil. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 370–375. [Google Scholar] [CrossRef]
- Ben Hassen-Trabelsi, A.; Kraiem, T.; Naoui, S.; Belayouni, H. Pyrolysis of waste animal fats in a fixed-bed reactor: Production and characterization of bio-oil and bio-char. Waste Manag. 2013, 34, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Meier, H.F.; Wiggers, V.R.; Zonta, G.R.; Scharf, D.R.; Simionatto, E.L.; Ender, L. A kinetic model for thermal cracking of waste cooking oil based on chemical lumps. Fuel 2015, 144, 50–59. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Peng, B.; Li, B.; Wang, H. Reduction kinetics for Fe2O3/NiO-doped compound in a methane atmosphere. J. Alloys Compd. 2017, 735, 365–371. [Google Scholar] [CrossRef]
- Periyasamy, B. Reaction pathway analysis in thermal cracking of waste cooking oil to hydrocarbons based on monomolecular lumped kinetics. Fuel 2015, 158, 479–487. [Google Scholar] [CrossRef]











| Element | C | H | O | N | S |
|---|---|---|---|---|---|
| Content (wt.%) | 83.52 | 13.88 | 1.25 | 1.22 | 0.13 |
| Composition | Content (wt.%) |
|---|---|
| Alkane oil | 90.98 |
| Ethylene glycol | 0.13 |
| Phthalate | 1.41 |
| Formic acid | 0.39 |
| Caprolactam | 0.37 |
| Polysiloxane | 6.25 |
| Moisture | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Li, B.; Wei, Y.; Zhou, S.; Wang, H. Cracking of Waste Engine Oil in the Presence of Fe3O4. Energies 2023, 16, 655. https://doi.org/10.3390/en16020655
Gao J, Li B, Wei Y, Zhou S, Wang H. Cracking of Waste Engine Oil in the Presence of Fe3O4. Energies. 2023; 16(2):655. https://doi.org/10.3390/en16020655
Chicago/Turabian StyleGao, Jialin, Bo Li, Yonggang Wei, Shiwei Zhou, and Hua Wang. 2023. "Cracking of Waste Engine Oil in the Presence of Fe3O4" Energies 16, no. 2: 655. https://doi.org/10.3390/en16020655