Managing the Effluents of Anaerobic Fermentations by Bioprocess Schemes Involving Membrane Bioreactors and Bio-Electrochemical Systems: A Mini-Review
Abstract
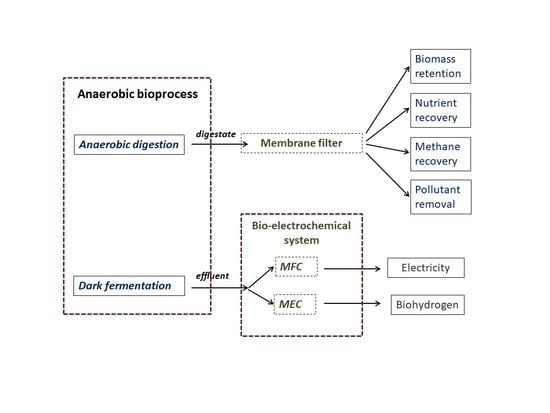
:1. Introduction
2. Methodology for Article Selection
3. Effluent Treatment of Biogas Production by Integrating Membranes with Anaerobic Digesters
3.1. AnMBR Configuration
3.2. AnMBR in Complex Systems
4. Bio-Electrochemical Systems (BES) for the Treatment of Dark Fermentative Hydrogen Production Effluents
- Simultaneous occurrence of different (and usually subsequent) phases of the redox processes in electrically linked distant spaces of the reactor, which, in practice, behaves as an additional stirring,
- Leveraging effect of the additional electric potential on the biochemical processes; this allows complementary endotherm reactions that would not occur in the absence of external potential,
- A supplementary selection advantage of electro-active microbial strains that increases the variety of organic compounds to biodegrade.
- Among the possible biological systems, the bio-electrochemical assistance to dark fermentation is reviewed in the following section.
4.1. BES Configurations
4.2. BES in Complex Systems
5. Conclusions
- To recover nutrients;
- To recover waste methane;
- To remove residual pollutants from anaerobic effluents;
- To increase the bioconversion efficiency of anaerobic systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.; Lee, D.W.; Cho, J. Application of direct contact membrane distillation process to treat anaerobic digestate. J. Membr. Sci. 2016, 511, 20–28. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Bertanza, G.; Abbà, A.; Sordi, M.; Pedrazzani, R. Synergy between anaerobic digestion and a post-treatment based on Thermophilic Aerobic Membrane Reactor (TAMR). Environ. Prog. Sustain. Energy 2017, 36, 1802–1809. [Google Scholar] [CrossRef]
- Rongwong, W.; Lee, J.; Goh, K.; Karahan, H.E.; Bae, T.-H. Membrane-based technologies for post-treatment of anaerobic effluents. NPJ Clean Water 2018, 1, 21. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Toward Resource Recovery from Wastewater: Extraction of Phosphorus from Digested Sludge Using a Hybrid forward Osmosis–Membrane Distillation Process. Environ. Sci. Technol. Lett. 2014, 1, 191–195. [Google Scholar] [CrossRef]
- Kuglarz, M.; Grűbel, K.; Bohdziewicz, J. Chemical precipitation and ammonia air stripping as effective pre-treatment methods before membrane filtration of co-digestion effluents. Desalination Water Treat. 2014, 55, 1672–1682. [Google Scholar] [CrossRef]
- Rózsenberszki, T.; Koók, L.; Bakonyi, P.; Nemestóthy, N.; Logroño, W.; Pérez, M.; Urquizo, G.; Recalde, C.; Kurdi, R.; Sarkady, A. Municipal waste liquor treatment via bio-electrochemical and fermentation (H2 + CH4) processes: Assessment of various technological sequences. Chemosphere 2017, 171, 692–701. [Google Scholar] [CrossRef]
- Bakonyi, P.; Dharmaraja, J.; Shobana, S.; Koók, L.; Rózsenberszki, T.; Nemestóthy, N.; Banu J, R.; Bélafi-Bakó, K.; Kumar, G. Leachate valorization in anaerobic biosystems: Towards the realization of waste-to-energy concept via biohydrogen, biogas and bio-electrochemical processes. Int. J. Hydrogen Energy 2019, 44, 17278–17296. [Google Scholar] [CrossRef]
- Bakonyi, P.; Kumar, G.; Koók, L.; Tóth, G.; Rózsenberszki, T.; Bélafi-Bakó, K.; Nemestothy, N. Microbial electrohydrogenesis linked to dark fermentation as integrated application for enhanced biohydrogen production: A review on process characteristics, experiences and lessons. Bioresour. Technol. 2018, 251, 381–389. [Google Scholar] [CrossRef]
- Waeger, F.; Delhaye, T.; Fuchs, W. The use of ceramic microfiltration and ultrafiltration membranes for particle removal from anaerobic digester effluents. Sep. Purif. Technol. 2010, 73, 271–278. [Google Scholar] [CrossRef]
- Kang, I.-J.; Yoon, S.-H.; Lee, C.-H. Comparison of the filtration characteristics of organic and inorganic membranes in a membrane-coupled anaerobic bioreactor. Water Res. 2002, 36, 1803–1813. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, H.; Qu, F.; Zhang, H.; Rong, H.; Yu, H.; Liang, H.; Ding, A.; Li, G.; Van der Bruggen, B. Application of membrane distillation to anaerobic digestion effluent treatment: Identifying culprits of membrane fouling and scaling. Sci. Total Environ. 2019, 688, 880–889. [Google Scholar] [CrossRef]
- Jacob, P.; Phungsai, P.; Fukushi, K.; Visvanathan, C. Direct contact membrane distillation for anaerobic effluent treatment. J. Membr. Sci. 2015, 475, 330–339. [Google Scholar] [CrossRef]
- Wen, C.; Huang, X.; Qian, Y. Domestic wastewater treatment using an anaerobic bioreactor coupled with membrane filtration. Process Biochem. 1999, 35, 335–340. [Google Scholar] [CrossRef]
- Zhang, J.; Padmasiri, S.; Fitch, M.; Norddahl, B.; Raskin, L.; Morgenroth, E. Influence of cleaning frequency and membrane history on fouling in an anaerobic membrane bioreactor. Desalination 2007, 207, 153–166. [Google Scholar] [CrossRef]
- Wu, B.; An, Y.; Li, Y.; Wong, F.S. Effect of adsorption/coagulation on membrane fouling in microfiltration process post-treating anaerobic digestion effluent. Desalination 2009, 242, 183–192. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, K.; Yu, H.; Liang, H.; Xie, B.; Li, G.; Qu, F.; van der Bruggen, B. Treatment of anaerobic digestion effluent using membrane distillation: Effects of feed acidification on pollutant removal, nutrient concentration and membrane fouling. Desalination 2019, 449, 6–15. [Google Scholar] [CrossRef]
- Annuar, A.M.; Nawi, N.I.M.; Bilad, M.R.; Jaafar, J.; Marbelia, L.; Nandianto, A.B.D. Improved bubbling for membrane fouling control in filtration of palm oil mill effluent anaerobic digester sludge. J. Water Process Eng. 2020, 36, 101350. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of organic loading rate on VFA production, organic matter removal and microbial activity of a two-stage thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef]
- Szelényi, G.; Bakonyi, P.; Nemestóthy, N. Aerobic stabilization of organic waste effluents from anaerobic treatment for agricultural use. Desalination Water Treat. 2020, 192, 424–430. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Kopsahelis, A.; Blika, P.; Paraskeva, C.; Lyberatos, G. Anaerobic digestion of olive mill wastewater in a periodic anaerobic baffled reactor (PABR) followed by further effluent purification via membrane separation technologies. J. Chem. Technol. Biotechnol. 2009, 84, 909–917. [Google Scholar] [CrossRef]
- Saddoud, A.; Sayadi, S. Application of acidogenic fixed-bed reactor prior to anaerobic membrane bioreactor for sustainable slaughterhouse wastewater treatment. J. Hazard. Mater. 2007, 149, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.P.; Merkle, W.; Tuczinski, M.; Saravia, F.; Horn, H.; Lemmer, A. Integration of membrane filtration in two-stage anaerobic digestion system: Specific methane yield potentials of hydrolysate and permeate. Bioresour. Technol. 2019, 275, 138–144. [Google Scholar] [CrossRef]
- Busato, C.J.; Da Ros, C.; Pellay, R.; Barbierato, P.; Pavan, P. Anaerobic membrane reactor: Biomethane from chicken manure and high-quality effluent. Renew. Energy 2020, 145, 1647–1657. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Sürmeli, R.Ö.; Calli, B. Dry anaerobic digestion of chicken manure coupled with membrane separation of ammonia. Bioresour. Technol. 2017, 244, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Dube, P.; Vanotti, M.; Szogi, A.; González, M.C.G. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wäeger-Baumann, F.; Fuchs, W. The Application of Membrane Contactors for the Removal of Ammonium from Anaerobic Digester Effluent. Sep. Sci. Technol. 2012, 47, 1436–1442. [Google Scholar] [CrossRef]
- Shi, X.; Zuo, J.; Zhang, M.; Wang, Y.; Yu, H.; Li, B. Enhanced biogas production and in situ ammonia recovery from food waste using a gas-membrane absorption anaerobic reactor. Bioresour. Technol. 2019, 292, 121864. [Google Scholar] [CrossRef]
- Jeong, E.; Kim, H.W.; Nam, J.-Y.; Shin, H.-S. Enhancement of bioenergy production and effluent quality by integrating optimized acidification with submerged anaerobic membrane bioreactor. Bioresour. Technol. 2010, 101, S7–S12. [Google Scholar] [CrossRef]
- Heile, S.; Chernicharo, C.; Brandt, E.; McAdam, E. Dissolved gas separation for engineered anaerobic wastewater systems. Sep. Purif. Technol. 2017, 189, 405–418. [Google Scholar] [CrossRef] [Green Version]
- Cookney, J.; Mcleod, A.; Mathioudakis, V.; Ncube, P.; Soares, A.; Jefferson, B.; McAdam, E. Dissolved methane recovery from anaerobic effluents using hollow fibre membrane contactors. J. Membr. Sci. 2016, 502, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Rongwong, W.; Goh, K.; Bae, T.-H. Energy analysis and optimization of hollow fiber membrane contactors for recovery of dissolve methane from anaerobic membrane bioreactor effluent. J. Membr. Sci. 2018, 554, 184–194. [Google Scholar] [CrossRef]
- Cheng, J.; Ye, Q.; Xu, J.; Yang, Z.; Zhou, J.; Cen, K. Improving pollutants removal by microalgae Chlorella PY-ZU1 with 15% CO2 from undiluted anaerobic digestion effluent of food wastes with ozonation pretreatment. Bioresour. Technol. 2016, 216, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Martinez, A.; Garcia, N.M.; Romero, I.; Seco, A.; Ferrer, J. Microalgae cultivation in wastewater: Nutrient removal from anaerobic membrane bioreactor effluent. Bioresour. Technol. 2012, 126, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Gong, W.; Yu, H.; Tang, X.; Yan, Z.; Luo, X.; Gan, Z.; Wang, T.; Li, G.; Liang, H. Immobilized microalgae for anaerobic digestion effluent treatment in a photobioreactor-ultrafiltration system: Algal harvest and membrane fouling control. Bioresour. Technol. 2018, 268, 139–148. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; He, N.; Zheng, Y.; Li, H.; Wang, H.; Wang, Y.; Lu, Y.; Li, Q.; Peng, Y. Nitrogen and phosphorus removal from anaerobically digested wastewater by microalgae cultured in a novel membrane photobioreactor. Biotechnol. Biofuels 2018, 11, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegrist, H.; Hunziker, W.; Hofer, H. Anaerobic digestion of slaughterhouse waste with UF-membrane separation and recycling of permeate after free ammonia stripping. Water Sci. Technol. 2005, 52, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, P.; Kumar, G.; Bélafi-Bakó, K.; Kim, S.-H.; Koter, S.; Kujawski, W.; Nemestóthy, N.; Peter, J.; Pientka, Z. A review of the innovative gas separation membrane bioreactor with mechanisms for integrated production and purification of biohydrogen. Bioresour. Technol. 2018, 270, 643–655. [Google Scholar] [CrossRef]
- Ojeda, F.; Bakonyi, P.; Buitrón, G. Improvement of methane content in a hydrogenotrophic anaerobic digester via the proper operation of membrane module integrated into an external-loop. Bioresour. Technol. 2017, 245, 1294–1298. [Google Scholar] [CrossRef]
- Wahid, R.; Mulat, D.G.; Gaby, J.C.; Horn, S.J. Effects of H2:CO2 ratio and H2 supply fluctuation on methane content and microbial community composition during in-situ biological biogas upgrading. Biotechnol. Biofuels 2019, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, P.; Nemestothy, N.; Simon, V.; Bélafi-Bakó, K. Review on the start-up experiences of continuous fermentative hydrogen producing bioreactors. Renew. Sustain. Energy Rev. 2014, 40, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Teng, S.-X.; Tong, Z.-H.; Li, W.-W.; Wang, S.-G.; Sheng, G.-P.; Shi, X.-Y.; Liu, X.-W.; Yu, H.-Q. Electricity generation from mixed volatile fatty acids using microbial fuel cells. Appl. Microbiol. Biotechnol. 2010, 87, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, S.B.; Srikanth, S.; Mohan, S.V.; Pant, D. Continuous mode operation of microbial fuel cell (MFC) stack with dual gas diffusion cathode design for the treatment of dark fermentation effluent. Int. J. Hydrogen Energy 2015, 40, 12424–12435. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Production of Electricity from Acetate or Butyrate Using a Single-Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.D.; Rader, G.; Regan, J.M.; Logan, B.E. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Bioresour. Technol. 2011, 102, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Aklujkar, M.; Krushkal, J.; DiBartolo, G.; Lapidus, A.; Land, M.L.; Lovley, D.R. The genome sequence of Geobacter metallireducens: Features of metabolism, physiology and regulation common and dissimilar to Geobacter sulfurreducens. BMC Microbiol. 2009, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Larios, A.L.; Solorza-Feria, O.; Vázquez-Huerta, G.; Esparza-García, F.; Rinderknecht-Seijas, N.; Poggi-Varaldo, H.M. Effects of architectural changes and inoculum type on internal resistance of a microbial fuel cell designed for the treatment of leachates from the dark hydrogenogenic fermentation of organic solid wastes. Int. J. Hydrogen Energy 2011, 36, 6199–6209. [Google Scholar] [CrossRef]
- Ren, Z.; Ward, T.E.; Regan, J.M. Electricity Production from Cellulose in a Microbial Fuel Cell Using a Defined Binary Culture. Environ. Sci. Technol. 2007, 41, 4781–4786. [Google Scholar] [CrossRef]
- Kim, C.; Song, Y.E.; Lee, C.R.; Jeon, B.-H.; Kim, J.R. Glycerol-fed microbial fuel cell with a co-culture of Shewanella oneidensis MR-1 and Klebsiella pneumonae J2B. J. Ind. Microbiol. Biotechnol. 2016, 43, 1397–1403. [Google Scholar] [CrossRef]
- Chookaew, T.; Prasertsan, P.; Ren, Z.J. Two-stage conversion of crude glycerol to energy using dark fermentation linked with microbial fuel cell or microbial electrolysis cell. New Biotechnol. 2014, 31, 179–184. [Google Scholar] [CrossRef]
- Goud, R.K.; Mohan, S.V. Pre-fermentation of waste as a strategy to enhance the performance of single chambered microbial fuel cell (MFC). Int. J. Hydrogen Energy 2011, 36, 13753–13762. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Ahn, Y.-H. Effectiveness of piggery waste treatment using microbial fuel cells coupled with elutriated-phased acid fermentation. Bioresour. Technol. 2017, 244, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Arslan, D.; Van Bogaert, G.; Alvarez-Gallego, Y.; De Wever, H.; Diels, L.; Vanbroekhoven, K. Integrated conversion of food waste diluted with sewage into volatile fatty acids through fermentation and electricity through a fuel cell. Environ. Technol. 2013, 34, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Ahmad, A.; Jafary, T.; Azad, A.K.; Kakooei, S.; Daud, W.R.W.; Sedighi, M. Assessment of immobilized cell reactor and microbial fuel cell for simultaneous cheese whey treatment and lactic acid/electricity production. Int. J. Hydrogen Energy 2017, 42, 9107–9115. [Google Scholar] [CrossRef]
- Rivera, I.; Bakonyi, P.; Cuautle-Marín, M.A.; Buitrón, G. Evaluation of various cheese whey treatment scenarios in single-chamber microbial electrolysis cells for improved biohydrogen production. Chemosphere 2017, 174, 253–259. [Google Scholar] [CrossRef]
- Wenzel, J.; Fuentes, L.; Cabezas, A.; Etchebehere, C. Microbial fuel cell coupled to biohydrogen reactor: A feasible technology to increase energy yield from cheese whey. Bioprocess Biosyst. Eng. 2017, 40, 807–819. [Google Scholar] [CrossRef]
- Huang, L.; Logan, B.E. Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl. Microbiol. Biotechnol. 2008, 80, 349–355. [Google Scholar] [CrossRef]
- Kim, T.; An, J.; Jang, J.K.; Chang, I.S. Coupling of anaerobic digester and microbial fuel cell for COD removal and ammonia recovery. Bioresour. Technol. 2015, 195, 217–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, M.; Yang, H.; Liang, L.; Gu, T. Microbial fuel cell hybrid systems for wastewater treatment and bioenergy production: Synergistic effects, mechanisms and challenges. Renew. Sustain. Energy Rev. 2019, 103, 13–29. [Google Scholar] [CrossRef]
- Oh, S.E.; Logan, B.E. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005, 39, 4673–4682. [Google Scholar] [CrossRef]
- Sharma, Y.; Li, B. Optimizing energy harvest in wastewater treatment by combining anaerobic hydrogen producing biofermentor (HPB) and microbial fuel cell (MFC). Int. J. Hydrogen Energy 2010, 35, 3789–3797. [Google Scholar] [CrossRef]
- Varanasi, J.L.; Roy, S.; Pandit, S.; Das, D. Improvement of energy recovery from cellobiose by thermophillic dark fermentative hydrogen production followed by microbial fuel cell. Int. J. Hydrogen Energy 2015, 40, 8311–8321. [Google Scholar] [CrossRef]
- Pandit, S.; Balachandar, G.; Das, D. Improved energy recovery from dark fermented cane molasses using microbial fuel cells. Front. Chem. Sci. Eng. 2014, 8, 43–54. [Google Scholar] [CrossRef]
- Song, Y.E.; El-Dalatony, M.M.; Kim, C.; Kurade, M.B.; Jeon, B.-H.; Kim, J.R. Harvest of electrical energy from fermented microalgal residue using a microbial fuel cell. Int. J. Hydrogen Energy 2019, 44, 2372–2379. [Google Scholar] [CrossRef]
- Sugnaux, M.; Happe, M.; Cachelin, C.P.; Gloriod, O.; Huguenin, G.; Blatter, M.; Fischer, F. Two stage bioethanol refining with multi litre stacked microbial fuel cell and microbial electrolysis cell. Bioresour. Technol. 2016, 221, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Mohan, S.V.; Fapyane, D.; Chang, I.S. Controlling Voltage Reversal in Microbial Fuel Cells. Trends Biotechnol. 2020, 38, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Chuda, A.; Ziemiński, K. Challenges in Treatment of Digestate Liquid Fraction from Biogas Plant. Performance of Nitrogen Removal and Microbial Activity in Activated Sludge Process. Energies 2021, 14, 7321. [Google Scholar] [CrossRef]









| Material | Type | Pore Size | Reference |
|---|---|---|---|
| Ceramic (αAl2O3 with active TiO2 layer) | Microfiltration | 200 nm | [9] |
| Ultrafiltration | 50 nm | ||
| Ultrafiltration | 20 kDa cut-off | ||
| PVDF | Microfiltration | 450 nm | [11] |
| Polyethylene | Ultrafiltration | 30 nm | [13] |
| Polyethersulphone | Ultrafiltration | 20 kDa cut-off | [14] |
| PVDF | Microfiltration | 220 nm | [15] |
| PVDF | Microfiltration | 450 nm | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szelényi, G.Z.; Kurdi, R.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Managing the Effluents of Anaerobic Fermentations by Bioprocess Schemes Involving Membrane Bioreactors and Bio-Electrochemical Systems: A Mini-Review. Energies 2022, 15, 1643. https://doi.org/10.3390/en15051643
Szelényi GZ, Kurdi R, Nemestóthy N, Bélafi-Bakó K, Bakonyi P. Managing the Effluents of Anaerobic Fermentations by Bioprocess Schemes Involving Membrane Bioreactors and Bio-Electrochemical Systems: A Mini-Review. Energies. 2022; 15(5):1643. https://doi.org/10.3390/en15051643
Chicago/Turabian StyleSzelényi, Gábor Z., Róbert Kurdi, Nándor Nemestóthy, Katalin Bélafi-Bakó, and Péter Bakonyi. 2022. "Managing the Effluents of Anaerobic Fermentations by Bioprocess Schemes Involving Membrane Bioreactors and Bio-Electrochemical Systems: A Mini-Review" Energies 15, no. 5: 1643. https://doi.org/10.3390/en15051643