Py-GC-MS Study on Catalytic Pyrolysis of Biocrude Obtained via HTL of Fruit Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
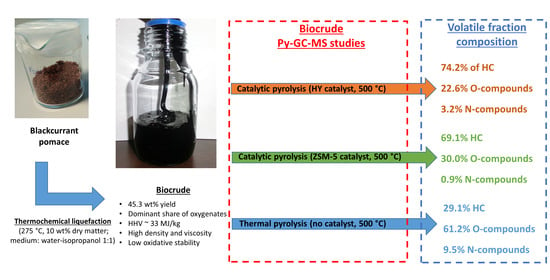
2.2. Biocrude Production via Thmochemical Liquefaction
2.3. Biocrude Characteristics
2.4. Fast Pyrolysis Tests—Py-GC-MS
3. Results and Discussion
3.1. Biocrude Production
3.2. Biocrude Composition and Properties
3.3. Biocrude Upgrading through Thermal and Catalytic Pyrolysis over ZSM-5 and HY Zeolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Krishna, B.B.; Mishra, G.; Kumar, J.; Bhaskar, T. Strategies for selection of thermo-chemical processes for the valorisation of biomass. Renew. Energy 2016, 98, 226–237. [Google Scholar] [CrossRef]
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A comprehensive state-of-technology review for upgrading bio-oil to renewable or blended hydrocarbon fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Li, C.; Aston, J.E.; Lacey, J.A.; Thompson, V.S.; Thompson, D.N. Impact of feedstock quality and variation on biochemical and thermochemical conversion. Renew. Sustain. Energy Rev. 2016, 65, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2011, 38, 68–94. [Google Scholar] [CrossRef]
- Perkins, G.; Bhaskar, T.; Konarova, M. Process development status of fast pyrolysis technologies for the manufacture of renewable transport fuels from biomass. Renew. Sustain. Energy Rev. 2018, 90, 292–315. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 848–889. [Google Scholar] [CrossRef]
- Dickerson, T.; Soria, J. Catalytic fast pyrolysis: A review. Energies 2013, 6, 514–538. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.W.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State-of-the- art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014, 40, 673–687. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Jakóbiec, J. Liquefaction of waste organic matter towards bio-oil in subcritical water. Przem. Chem. 2017, 96, 1913–1918. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Eurostat. The Fruit and Vegetable Sector in the EU—A Statistical Overview; Eurostat: Luxemburg, 2019. [Google Scholar]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Saber, M.; Nakhshiniev, B.; Yoshikawa, K. A review of production and upgrading of algal bio-oil. Renew. Sustain. Energy Rev. 2016, 58, 918–930. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Jacobson, K.; Maheria, K.C.; Kumar Dalai, A. Bio-oil valorization: A review. Renew. Sustain. Energy Rev. 2013, 23, 91–106. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Vos, M.P.; Brilman, D.W.F. Effect of process conditions on bio-oil obtained through continuous hydrothermal liquefaction of Scenedesmus sp. microalgae. J. Anal. Appl. Pyrolysis 2018, 134, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Wadrzyk, M.; Berdel, M.; Janus, R.; Brilman, D.W.F. Hydrothermal processing of pine wood: Effect of process variables on bio-oil quality and yield. E3S Web Conf. 2019, 108, 02004. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Julson, J. Application, deactivation, and regeneration of heterogeneous catalysts in bio-oil upgrading. Catalysts 2016, 6, 195. [Google Scholar] [CrossRef]
- Montesantos, N.; Maschietti, M. Supercritical carbon dioxide extraction of lignocellulosic bio-oils: The potential of fuel upgrading and chemical recovery. Energies 2020, 13, 1600. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and opportunities for bio-oil refining: A review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Korzeniowski, Ł.; Plata, M.; Janus, R.; Lewandowski, M.; Maziarka, P. Valorization of Blackcurrant Pomace through Thermochemical Liquefaction in Mixed Solvents. In Proceedings of the 34th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Taormina, Italy, 28 June–2 July 2021; pp. 1–10. [Google Scholar]
- Deniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Optimisation of bio-oil production by hydrothermal liquefaction of agro-industrial residues: Blackcurrant pomace (Ribes nigrum L.) as an example. Biomass Bioenergy 2016, 95, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Anouti, S.; Haarlemmer, G.; Déniel, M.; Roubaud, A. Analysis of Physicochemical Properties of Bio-Oil from Hydrothermal Liquefaction of Blackcurrant Pomace. Energy Fuels 2016, 30, 398–406. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Grzywacz, P.; Janus, R.; Michalik, M. A two-stage processing of cherry pomace via hydrothermal treatment followed by biochar gasification. Renew. Energy 2021, 179, 248–261. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Arauzo, P.J.; Wądrzyk, M.; Kruse, A. Py-GC-MS of hydrochars produced from brewer’s spent grains. J. Anal. Appl. Pyrolysis 2019, 140, 255–263. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, Y.T.; Vispute, T.P.; Xiao, R.; Huber, G.W. Catalytic conversion of biomass-derived feedstocks into olefins and aromatics with ZSM-5: The hydrogen to carbon effective ratio. Energy Environ. Sci. 2011, 4, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Agblevor, F. Separation and Hydroprocessing of HZSM-5 Catalytic Olive Mill Waste Sludge Bio-oil. Energy Fuels 2016, 30, 10524–10533. [Google Scholar] [CrossRef]
- Brilman, D.W.F.; Drabik, N.; Wądrzyk, M. Hydrothermal co-liquefaction of microalgae, wood, and sugar beet pulp. Biomass Convers. Biorefinery 2017, 7, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Wądrzyk, M.; Janus, R.; Lewandowski, M.; Magdziarz, A. On mechanism of lignin decomposition—Investigation using microscale techniques: Py-GC-MS, Py-FT-IR and TGA. Renew. Energy 2021, 177, 942–952. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]





| Element/Parameter a | Unit | Feedstock | HTL Biocrude | ||
|---|---|---|---|---|---|
| C | wt% | 50.54 | ±0.67 | 69.25 | ±0.53 |
| H | wt% | 7.13 | ±0.15 | 9.11 | ±0.14 |
| N | wt% | 2.72 | ±0.17 | 2.38 | ±0.22 |
| S | wt% | 0.22 | ±0.06 | n.d. | - |
| O | wt% | 36.57 | ±0.82 | 19.25 | ±0.47 |
| H/C | mol mol−1 | 1.679 | ±0.013 | 1.579 | ±0.033 |
| O/C | mol mol−1 | 0.543 | ±0.019 | 0.209 | ±0.007 |
| HHV | MJ kg−1 | 20.78 | ±0.59 | 33.07 | ±0.26 |
| Gas Components | |||||||
|---|---|---|---|---|---|---|---|
| CO2 | CO | H2 | CH4 | C2H6 | C3H6 | C3H8 | |
| Mean value (wt%) | 87.21 | 5.47 | 0.32 | 0.33 | 0.04 | 6.55 | 0.08 |
| SD | ±0.02 | ±0.01 | ±0.01 | ±0.02 | ±0.01 | ±0.03 | 0 |
| Group | RT (min) | Name | Formula | Relative Area (%) |
|---|---|---|---|---|
| Acids | 5.7 | Propanoic acid | C3H6O2 | 0.32 |
| 60.4 | n-Hexadecanoic acid | C16H32O2 | 8.22 | |
| 66.6 | 9-Octadecenoic acid | C18H34O2 | 15.39 | |
| 67.2 | 9,12-octadecadienoic acid | C18H32O2 | 28.51 | |
| 67.9 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | C18H30O2 | 3.04 | |
| Esters | 12.6 | Isopropyl 2-hydroxypropanoate | C6H12O3 | 1.08 |
| 30.5 | Pentanoic acid, 4-oxo-, propyl ester | C8H14O3 | 0.48 | |
| 60.6 | Hexadecanoic acid isopropyl ester | C19H38O2 | 3.60 | |
| 64.9 | 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | 0.73 | |
| 66.3 | Octadecanoic acid isopropyl ester | C21H42O2 | 3.79 | |
| 66.9 | 9,12-Octadecadienoic acid isopropyl ester | C21H38O2 | 7.52 | |
| 67.7 | 9,12,15-Octadecatrienoic acid, isopropyl ester | C21H36O2 | 1.63 | |
| Phenol deriv. | 24.8 | Benzene, ethoxy- | C8H10O | 0.50 |
| 30.8 | Phenol, 2-methoxy- | C7H8O2 | 0.68 | |
| 34.1 | Phenol, 4-ethyl- | C8H10O | 0.69 | |
| 39.1 | Phenol, 4-ethyl-2-methoxy- | C9H12O2 | 0.57 | |
| Other O-cmpd | 25.1 | Butyrolactone | C4H6O2 | 0.41 |
| 27.7 | 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | C6H8O2 | 0.36 | |
| 74.5 | 2,3-Butanediol, diacetate | C8H14O4 | 0.55 | |
| N-cmpd | 15.1 | Pyrazine, methyl- | C5H6N2 | 0.64 |
| 33.1 | 3-Pyridinol | C5H5NO | 4.75 | |
| 33.8 | 2-Pyrrolidinone | C4H7NO | 1.04 | |
| 35.2 | 3-Pyridinol, 6-methyl- | C6H7NO | 0.74 | |
| 50.3 | N-Ethyl-2-isopropoxycarbonylazetidine | C9H17NO2 | 2.29 | |
| 61.4 | 1,2,5-Oxadiazole-3,4-dicarboxamide | C4H4N4O3 | 0.86 | |
| 64.3 | 3,6-Diisopropylpiperazin-2,5-dione | C10H18N2O2 | 0.73 | |
| Complex cmpd | 69.7 | l-Valine, n-propargyloxycarbonyl-, pentadecyl ester | C24H43NO4 | 0.72 |
| 70.4 | Benzenamine, N-(4-pyridinylmethylene)- | C12H10N2 | 0.56 | |
| 71.5 | 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolopyrazine | C14H22N2O2 | 0.80 | |
| 71.6 | 9H-Carbazole, 9-(1-naphthalenyl)- | C22H15N | 0.23 | |
| 85.2 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- | C14H16N2O2 | 1.15 |
| Parameter | Unit | HTL Biocrude a | Diesel Oil b | Heavy Heating Oil c |
|---|---|---|---|---|
| Density, 15 °C | kg m−3 | 1114 ± 17 | 820–845 | >890 |
| Kinematic viscosity, 40 °C | mm2 s−1 | 558 ± 20 | 2–4.5 | — |
| Dynamic viscosity, 40 °C | cP | 625 ± 16 | — | <800 e |
| Oxidative stability, 140 °C | min | 29.81 ± 0.15 | 67 a | — |
| Water content | wt% | 0.08 ± 0.03 | 0.02 | <1.0 |
| pH | — | 4.15 ± 0.09 | — | — |
| TAN | mg NaOH g−1 | 80 ± 4 | — | — |
| Solids content | wt% | 10.21 ± 1.77 d | 0.0024 | <0.5 |
| Ash content | wt% | 0.07 ± 0.01 | — | <0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wądrzyk, M.; Plata, M.; Zaborowska, K.; Janus, R.; Lewandowski, M. Py-GC-MS Study on Catalytic Pyrolysis of Biocrude Obtained via HTL of Fruit Pomace. Energies 2021, 14, 7288. https://doi.org/10.3390/en14217288
Wądrzyk M, Plata M, Zaborowska K, Janus R, Lewandowski M. Py-GC-MS Study on Catalytic Pyrolysis of Biocrude Obtained via HTL of Fruit Pomace. Energies. 2021; 14(21):7288. https://doi.org/10.3390/en14217288
Chicago/Turabian StyleWądrzyk, Mariusz, Marek Plata, Kamila Zaborowska, Rafał Janus, and Marek Lewandowski. 2021. "Py-GC-MS Study on Catalytic Pyrolysis of Biocrude Obtained via HTL of Fruit Pomace" Energies 14, no. 21: 7288. https://doi.org/10.3390/en14217288