Co-Ensiling of Wheat Straw as an Alternative Pre-Treatment to Chemical, Hydrothermal and Mechanical Methods for Methane Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomasses
2.2. Co-Ensiling Setup
2.3. Pre-Treatment of Biomasses
2.4. Biochemical Methane Potential Assays
2.5. Sample Analysis
2.6. Data Analysis
- y(t) = The cumulative methane yield after a specific digestion period, t, in days (NL CH4 kg−1 VS)
- y0 = The ultimate methane potential (BMP) (NL CH4 kg−1 VS)
- k = The hydrolysis constant (day−1)
- t = Cumulative digestion time for biogas production (days)
- y(t) = The cumulative methane yield after a specific digestion period, t, in days (NL CH4 kg−1 VS)
- A = The biogas production potential (NL CH4 kg−1 VS)
- µm = The maximum biogas production rate (NL CH4 kg−1 VS day−1)
- λ = Lag phase period (days)
- t = Cumulative digestion time for biogas production (days)
3. Results and Discussion
3.1. Dry Matter Losses
3.2. Effect of Co-Ensiling
3.3. Mechanical, Hydrothermal and Chemical Pre-Treatment Effects
3.4. Competitiveness of Co-Ensiling as a Pre-Treatment
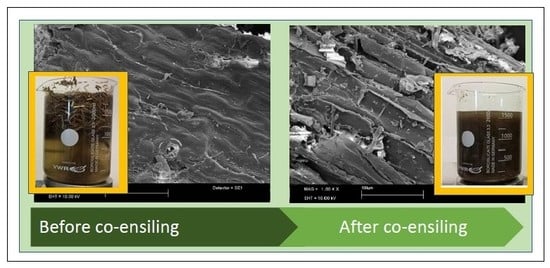
3.5. Surface Topography Analysis of Pre-Treated Straw
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. European Strategy: Innovating for Sustainable Growth: A Bioeconomy for Europe; Publications Office of the European Union: Luxembourg, Belgium, 2012. [Google Scholar]
- Selvaggi, R.; Valenti, F.; Pappalardo, G.; Rossi, L.; Bozzetto, S.; Pecorino, B.; Dale, B.E. Sequential crops for food, energy, and economic development in rural areas: The case of Sicily. Biofuels Bioprod. Biorefin. 2018, 12, 22–28. [Google Scholar] [CrossRef]
- Fjørtoft, K.; Morken, J.; Hanssen, J.F.; Briseid, T. Pre-treatment methods for straw for farm-scale biogas plants. Biomass Bioenergy 2019, 124, 88–94. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaggi, R.; Pappalardo, G.; Chinnici, G.; Fabbri, C.I. Assessing land efficiency of biomethane industry: A case study of Sicily. Energy Policy 2018, 119, 689–695. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, X.; Li, Z.; Wang, X.; Sun, J. Effects of liquid digestate pretreatment on biogas production for anaerobic digestion of wheat straw. Bioresour. Technol. 2019, 280, 345–351. [Google Scholar] [CrossRef]
- Suseela, V. Potential roles of plant biochemistry in mediating ecosystem responses to warming and drought. In Ecosystem Consequences of Soil Warming; Academic Press: Cambridge, MA, USA, 2019; pp. 103–124. [Google Scholar]
- Laureano-Perez, L.; Teymouri, F.; Alizadeh, H.; Dale, B.E. Understanding Factors that Limit Enzymatic Hydrolysis of Biomass. Appl. Biochem. Biotechnol. 2005, 124, 1081–1099. [Google Scholar] [CrossRef]
- Koullas, D.P.; Christakopoulos, P.; Kekos, D.; Macris, B.J.; Koukios, E.G. Correlating the effect of pretreatment on the enzymatic hydrolysis of straw. Biotechnol. Bioeng. 1992, 39, 113–116. [Google Scholar] [CrossRef]
- Bai, X.; Wang, G.; Yu, Y.; Wang, D.; Wang, Z. Changes in the physicochemical structure and pyrolysis characteristics of wheat straw after rod-milling pretreatment. Bioresour. Technol. 2018, 250, 770–776. [Google Scholar] [CrossRef]
- Irmak, S.; Meryemoglu, B.; Sandip, A.; Subbiah, J.; Mitchell, R.B.; Sarath, G. Microwave pretreatment effects on switchgrass and miscanthus solubilization in subcritical water and hydrolysate utilization for hydrogen production. Biomass Bioenergy 2018, 108, 48–54. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.L.; Gu, Y.; Liu, Z.; Shen, Z.; Chu, H.; Zhou, X. Enhancing methane production from rice straw by extrusion pretreatment. Appl. Energy 2014, 122, 34–41. [Google Scholar] [CrossRef]
- Zeynali, R.; Khojastehpour, M.; Ebrahimi-Nik, M. Effect of ultrasonic pre-treatment on biogas yield and specific energy in anaerobic digestion of fruit and vegetable wholesale market wastes. Sustain. Environ. Res. 2017, 27, 259–264. [Google Scholar] [CrossRef]
- Bolado-Rodríguez, S.; Toquero, C.; Martín-Juárez, J.; Travaini, R.; García-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.B.; Gogate, P.R. Alkaline and ultrasound assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrason. Sonochem. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.H.; Horn, S.J. Effect of different steam explosion conditions on methane potential and enzymatic saccharification of birch. Bioresour. Technol. 2013, 127, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Jiang, W.; Jia, J.X.; Xu, J. PH pre-corrected liquid hot water pretreatment on corn stover with high hemicellulose recovery and low inhibitors formation. Bioresour. Technol. 2014, 153, 292–299. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Wu, D.; Zhang, P.; Wang, H.; Tao, X.; Ye, J.; Nabi, M. Enzyme Pretreatment Enhancing Biogas Yield from Corn Stover: Feasibility, Optimization, and Mechanism Analysis. J. Agric. Food Chem. 2018, 66, 10026–10032. [Google Scholar] [CrossRef]
- Larsen, S.U.; Hjort-Gregersen, K.; Vazifehkhoran, A.H.; Triolo, J.M. Co-ensiling of straw with sugar beet leaves increases the methane yield from straw. Bioresour. Technol. 2017, 245, 106–115. [Google Scholar] [CrossRef]
- Pakarinen, A.; Maijala, P.; Jaakkola, S.; Stoddard, F.; Kymäläinen, M.; Viikari, L. Evaluation of preservation methods for improving biogas production and enzymatic conversion yields of annual crops. Biotechnol. Biofuels 2011, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Perschke, Y.M.L.; Fontaine, D.; Ward, A.J.; Eriksen, J.; Sørensen, P.; Møller, H.B. Co-ensiling of cover crops and barley straw for biogas production. Renew. Energy 2019, 142, 677–683. [Google Scholar] [CrossRef]
- Ni, K.; Wang, Y.; Cai, Y.; Pang, H. Natural lactic acid bacteria population and silage fermentation of whole-crop wheat. Asian Australas. J. Anim. Sci. 2015, 28, 1123–1132. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.R.A.V.; Do Carmo, M.S.; Melo, B.O.; Alves, M.S.; Dos Santos, C.I.; Monteiro, S.G.; Bomfim, M.R.Q.; Fernandes, E.S.; Monteiro-Neto, V. In vitro antimicrobial activity and probiotic potential of bifidobacterium and lactobacillus against species of clostridium. Nutrients 2019, 11, 448. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.; Heiermann, M.; Idler, C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour. Technol. 2011, 102, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, O.; Lehtomäki, A.; Rissanen, S.; Rintala, J. Storing energy crops for methane production: Effects of solids content and biological additive. Bioresour. Technol. 2008, 99, 7074–7082. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ge, X.; Xu, F.; Li, Y. Effect of total solids content on giant reed ensilage and subsequent anaerobic digestion. Process Biochem. 2016, 51, 73–79. [Google Scholar] [CrossRef]
- Ahmed, S.; Einfalt, D.; Kazda, M. Co-Digestion of Sugar Beet Silage Increases Biogas Yield from Fibrous Substrates. Biomed Res. Int. 2016, 2016, 2147513. [Google Scholar] [CrossRef] [Green Version]
- Kryvoruchko, V.; Machmüller, A.; Bodiroza, V.; Amon, B.; Amon, T. Anaerobic digestion of by-products of sugar beet and starch potato processing. Biomass Bioenergy 2009, 33, 620–627. [Google Scholar] [CrossRef]
- Strausbaugh, C.A.; Gillen, A.M. Bacteria and yeast associated with sugar beet root rot at harvest in the intermountain west. Plant Dis. 2008, 92, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Johnson, H.E.; Merry, R.J.; Davies, D.R.; Kell, D.B.; Theodorou, M.K.; Griffith, G.W. Vacuum packing: A model system for laboratory-scale silage fermentations. J. Appl. Microbiol. 2005, 98, 106–113. [Google Scholar] [CrossRef]
- Vazifehkhoran, A.H.; Shin, S.G.; Triolo, J.M. Use of tannery wastewater as an alternative substrate and a pre-treatment medium for biogas production. Bioresour. Technol. 2018, 258, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Banoth, C.; Sunkar, B.; Tondamanati, P.R.; Bhukya, B. Improved physicochemical pretreatment and enzymatic hydrolysis of rice straw for bioethanol production by yeast fermentation. 3 Biotech 2017, 7, 334. [Google Scholar] [CrossRef]
- VDI (Verein Deutscher Ingenieure). Fermentation of Organic Materials Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Verein Deutscher Ingenieure: Düsseldorf, Germany, 2006. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- U.S. EPA. METHOD 1683 Total, Fixed and Volatile, Solids and Biosolids. Sci. Technol. 2001. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1002CZ8.txt (accessed on 3 August 2020).
- Vahlberg, C.; Nordell, E.; Wiberg, L.; Schnürer, A. Method for Correction of VFA Loss in Determination of Dry Matter in Biomass; Svenskt Gastekniskt Center: Malmo, Sweden, 2013. [Google Scholar]
- Vazifehkhoran, A.H.; Triolo, J.M.; Larsen, S.U.; Stefanek, K.; Sommer, S.G. Assessment of the variability of biogas production from sugar beet silage as affected by movement and loss of the produced alcohols and organic acids. Energies 2016, 9, 368. [Google Scholar] [CrossRef] [Green Version]
- Symons, G.E.; Buswell, A.M. The Methane Fermentation of Carbohydrates. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]
- Vazifehkhoran, A.H.; Triolo, J.M. A novel mathematical modelling of waste biomass decomposition to facilitate rapid methane potential prediction. J. Clean. Prod. 2019, 220, 1222–1230. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Bah, H.; Zhang, W.; Wu, S.; Qi, D.; Kizito, S.; Dong, R. Evaluation of batch anaerobic co-digestion of palm pressed fiber and cattle manure under mesophilic conditions. Waste Manag. 2014, 34, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Pearson: London, UK, 2011; ISBN 0582219272. [Google Scholar]
- Ambye-Jensen, M.; Thomsen, S.T.; Kádár, Z.; Meyer, A.S. Ensiling of wheat straw decreases the required temperature in hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuger, E.; Nges, I.; Björnsson, L. Ensiling of crops for biogas production: Effects on methane yield and total solids determination. Biotechnol. Biofuels 2011, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Liu, Y.; Uchida, S.; Kawarada, K.; Ukagami, Y.; Ichinose, H.; Kaneko, S.; Fukuda, K. Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci. Biotechnol. Biochem. 2008, 72, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Cegrí, V.; Ángeles de la Rubia, M.; Raposo, F.; Borja, R. Effect of hydrothermal pretreatment of sunflower oil cake on biomethane potential focusing on fibre composition. Bioresour. Technol. 2012, 123, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dutt, D.; Tyagi, C.H. Complete characterization of wheat straw (Triticum aestivum PBW-343 L. Emend. Fiori & PAOL.)—A renewable source of fibres for pulp and paper making. BioResources 2011, 6, 154–177. [Google Scholar]
- Yao, Y.; Chen, S. A novel and simple approach to the good process performance of methane recovery from lignocellulosic biomass alone. Biotechnol. Biofuels 2016, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef] [Green Version]
- Barman, D.N.; Haque, M.A.; Kang, T.H.; Kim, M.K.; Kim, J.; Kim, H.; Yun, H.D. Alkali pretreatment of wheat straw (Triticum aestivum) at boiling temperature for producing a bioethanol precursor. Biosci. Biotechnol. Biochem. 2012, 76, 120480. [Google Scholar] [CrossRef] [Green Version]






| Mixing Ratio (WW-Based) (Sugar Beet, Root:Straw) | 100:0 | 94:6 | 88:12 | 80:20 | 0:100 |
|---|---|---|---|---|---|
| Ensiling duration (2, 6 or 8 months) | 2 6 8 | 2 6 8 | 2 6 8 | 2 6 8 | 0 |
| Pre-treatments (M, HT, CT or CCT) | 8-HT 8-CT 8-CCT | 0-M 0-HT 0-CT 0-CCT |
| Parameters | Units | 94:6 | 88:12 | 80:20 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ensiling Period | Months | 2 | 6 | 8 | 2 | 6 | 8 | 2 | 6 | 8 |
| Solid analysis: | ||||||||||
| Dry matter | g kg−1 | 246.5 ± 16.4 | 177.7 ± 7.6 | 240.5 ± 2.4 | 260.5 ± 8.8 | 271.2 ± 6.9 | 282.0 ± 5.7 | 350.0 ± 1.2 | 338.8 ± 9.2 | 313.9 ± 4.4 |
| Volatile solids | g kg−1 | 232.9 ± 15.8 | 163.5 ± 6.4 | 226.8 ± 3.0 | 244.5 ± 8.0 | 252.2 ± 4.4 | 262.8 ± 5.2 | 324.5 ± 0.7 | 313.4 ± 9.9 | 279.8 ± 16.6 |
| Volatile fatty acids *: | ||||||||||
| Acetic acid | g L−1 | 0.66 ± 0.01 | 0.91 ± 0.01 | 0.85 ± 0.03 | 0.68 ± 0.02 | 0.77 ± 0.02 | 0.74 ± 0.01 | 0.68 ± 0.02 | 0.84 ± 0.02 | 0.92 ± 0.00 |
| Butyric acid | g L−1 | 0.13 ± 0.01 | 0.00 ± 0.00 | 0.10 ± 0.00 | 0.12 ± 0.00 | 0.07 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.10 ± 0.01 | 0.09 ± 0.00 |
| Alcohols: | ||||||||||
| Ethanol | g L−1 | 0.03 ± 0.00 | 0.54 ± 0.00 | 0.43 ± 0.00 | 0.12 ± 0.00 | 0.09 ± 0.00 | 0.07 ± 0.00 | 0.12 ± 0.00 | 0.34 ± 0.00 | 0.36 ± 0.00 |
| Glycerol | g L−1 | 0.11 ± 0.00 | 0.94 ± 0.05 | 0.43 ± 0.01 | 0.12 ± 0.00 | 0.09 ± 0.00 | 0.07 ± 0.00 | 0.12 ± 0.00 | 0.34 ± 0.00 | 0.36 ± 0.00 |
| Sorbitol | g L−1 | 11.89 ± 0.01 | 6.20 ± 0.06 | 14.50 ± 0.06 | 11.58 ± 0.02 | 9.54 ± 0.03 | 14.26 ± 0.00 | 11.93 ± 0.01 | 12.55 ± 0.02 | 10.91 ± 0.00 |
| Sugars: | ||||||||||
| Maltose | g L−1 | 0.89 ± 0.00 | 0.37 ± 0.05 | 0.98 ± 0.00 | 1.01 ± 0.00 | 0.75 ± 0.00 | 1.21 ± 0.00 | 1.19 ± 0.00 | 1.01 ± 0.00 | 0.96 ± 0.00 |
| Glucose | g L−1 | 5.11 ± 0.00 | 2.14 ± 0.03 | 5.88 ± 0.06 | 5.93 ± 0.01 | 4.24 ± 0.03 | 7.13 ± 0.00 | 7.17 ± 0.00 | 6.52 ± 0.00 | 6.23 ± 0.00 |
| Elemental composition: | ||||||||||
| Carbon | wt% | 58.38 | 69.63 | 58.63 | 56.37 | 60.73 | 61.36 | 56.38 | 56.90 | 61.88 |
| Nitrogen | wt% | 1.43 | 1.57 | 1.51 | 1.20 | 1.02 | 0.95 | 1.21 | 0.96 | 1.00 |
| Oxygen | wt% | 33.83 | 23.37 | 34.35 | 35.09 | 31.57 | 30.46 | 34.84 | 35.61 | 30.21 |
| Sulphur | wt% | 0.97 | 0.85 | 0.77 | 0.74 | 0.53 | 0.43 | 0.44 | 0.43 | 0.35 |
| Hydrogen | wt% | 5.39 | 4.59 | 4.75 | 6.60 | 6.14 | 6.80 | 7.13 | 6.20 | 6.56 |
| Parameters | Units | 100:0 | 0:100 | ||
|---|---|---|---|---|---|
| Ensiling Period | Months | 2 | 6 | 8 | 2 |
| Solid analysis: | |||||
| Dry matter | g kg−1 | 196.1 ± 7.1 | 162.4 ± 3.8 | 139.0 ± 0.4 | 915.6 ± 0.5 |
| Volatile solids | g kg−1 | 187.1 ± 6.6 | 153.5 ± 3.6 | 130.6 ± 0.2 | 848.6 ± 2.9 |
| Volatile fatty acids*: | |||||
| Acetic acid | g L−1 | 0.82 ± 0.04 | 0.76 ± 0.02 | 1.26 ± 0.03 | N/A |
| Butyric acid | g L−1 | 0.08 ± 0.01 | 0.03 ± 0.01 | 0.00 ± 0.00 | N/A |
| Alcohols: | |||||
| Ethanol | g L−1 | 0.10 ± 0.00 | 0.40 ± 0.00 | 0.68 ± 0.00 | N/A |
| Glycerol | g L−1 | 0.11 ± 0.00 | 0.20 ± 0.01 | 0.75 ± 0.01 | N/A |
| Sorbitol | g L−1 | 10.23 ± 0.07 | 6.14 ± 0.00 | 3.66 ± 0.00 | N/A |
| Sugars: | |||||
| Maltose | g L−1 | 0.40 ± 0.06 | 0.37 ± 0.00 | 0.22 ± 0.00 | N/A |
| Glucose | g L−1 | 1.66 ± 0.10 | 1.69 ± 0.05 | 1.17 ± 0.02 | N/A |
| Elemental composition: | |||||
| Carbon | wt% | 49.37 | 51.32 | 54.11 | 64.24 |
| Nitrogen | wt% | 1.21 | 1.37 | 1.68 | 1.09 |
| Oxygen | wt% | 41.35 | 41.12 | 38.71 | 28.51 |
| Sulphur | wt% | 3.19 | 1.78 | 1.52 | 0.16 |
| Hydrogen | wt% | 4.87 | 4.41 | 3.98 | 5.98 |
| Sample | Ensiling Duration | Additional Pre-Treatment | BMP | Biodegradability | Synergistic Effect of Co-Ensiling and Pre-Treatment | Kinetic Constants | |||
|---|---|---|---|---|---|---|---|---|---|
| k | A | µm | λ | ||||||
| [Months] | [NL CH4 kg−1 VS] | [%] | [%] | [day−1] | [NL CH4 kg−1 VS] | [NL CH4 kg−1 VS day−1] | [day] | ||
| 100:0 | 2 | 369.4 ± 7.4 | 84.0 | 0.24 | |||||
| 100:0 | 6 | 412.5 ± 5.6 | 92.2 | 0.25 | |||||
| 100:0 | 8 | 443.6 ± 11.4 | 94.7 | 0.21 | |||||
| 94:06 | 2 | 304.5 ± 10.2 | 53.7 | −3.2 | 0.21 | ||||
| 94:06 | 6 | 411.1 ± 2.6 | 60.0 | 21.8 | 0.18 | ||||
| 94:06 | 8 | 354.1 ± 15.1 | 64.4 | 1.2 | 0.17 | ||||
| 88:12 | 2 | 291.2 ± 12.8 | 50.2 | 8.8 | 0.20 | ||||
| 88:12 | 6 | 307.4 ± 0.4 | 49.5 | 10.6 | 0.22 | ||||
| 88:12 | 8 | 312.9 ± 11.8 | 48.1 | 11.6 | 0.16 | ||||
| 80:20 | 2 | 253.9 ± 11.9 | 42.6 | 13.8 | 0.19 | ||||
| 80:20 | 6 | 244.3 ± 9.3 | 42.6 | 8.5 | 0.18 | ||||
| 80:20 | 8 | 298.6 ± 18.0 | 46.0 | 34.7 | 0.14 | ||||
| 80:20 | 8 | HT | 310.5 ± 8.3 | 47.9 | 4.0 | 309.32 | 20.28 | 0.00 | |
| 80:20 | 8 | CT | 298.2 ± 24.9 | 46.0 | −0.1 | 298.03 | 20.88 | 0.00 | |
| 80:20 | 8 | CCT | 312.6 ± 2.6 | 48.2 | 4.7 | 308.91 | 20.88 | 0.00 | |
| 0:100 | 0 | 186.7 ± 12.0 | 28.3 | 0.04 | 190.99 | 8.83 | 0.64 | ||
| 0:100 | 0 | M | 186.6 ± 6.2 | 28.2 | −3.2 | 174.55 | 14.98 | 2.14 | |
| 0:100 | 0 | HT | 214.8 ± 6.5 | 32.4 | 15.1 | 205.50 | 15.48 | 1.94 | |
| 0:100 | 0 | CT | 223.4 ± 5.8 | 33.9 | 19.7 | 214.04 | 16.81 | 2.19 | |
| 0:100 | 0 | CCT | 234.1 ± 5.9 | 35.5 | 25.4 | 224.33 | 16.81 | 1.87 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieborg, M.U.; Jønson, B.D.; Larsen, S.U.; Vazifehkhoran, A.H.; Triolo, J.M. Co-Ensiling of Wheat Straw as an Alternative Pre-Treatment to Chemical, Hydrothermal and Mechanical Methods for Methane Production. Energies 2020, 13, 4047. https://doi.org/10.3390/en13164047
Sieborg MU, Jønson BD, Larsen SU, Vazifehkhoran AH, Triolo JM. Co-Ensiling of Wheat Straw as an Alternative Pre-Treatment to Chemical, Hydrothermal and Mechanical Methods for Methane Production. Energies. 2020; 13(16):4047. https://doi.org/10.3390/en13164047
Chicago/Turabian StyleSieborg, Mads Ujarak, Brian Dahl Jønson, Søren Ugilt Larsen, Ali Heidarzadeh Vazifehkhoran, and Jin Mi Triolo. 2020. "Co-Ensiling of Wheat Straw as an Alternative Pre-Treatment to Chemical, Hydrothermal and Mechanical Methods for Methane Production" Energies 13, no. 16: 4047. https://doi.org/10.3390/en13164047