Advanced Wastewater Treatment to Eliminate Organic Micropollutants in Wastewater Treatment Plants in Combination with Energy-Efficient Electrolysis at WWTP Mainz
Abstract
:1. Introduction and Background
1.1. Organic Micropollutants (OMP) in Wastewater
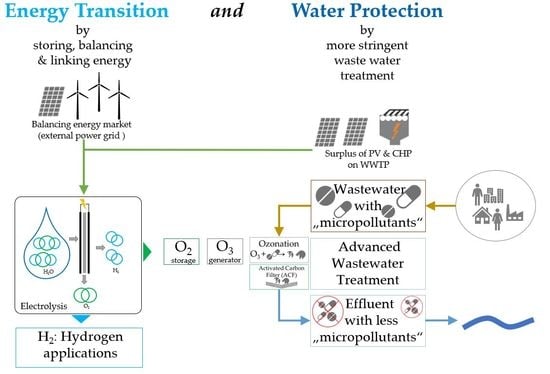
1.2. Sector Coupling and Energy Transition
2. WWTP Mainz
2.1. Description of the Plant
2.2. Organic Micropollutants
- the limited sampling only shows a snapshot and is not statistically assured;
- deviation between the concentrations found in Mainz and on other WWTPs might depend on regional differences in the application and prescription of substances.
2.3. Energetic Situation and Upcoming Changes
3. Practical Implementation: The “Arrived” Project
3.1. Integrated Concept
3.2. Objectives and Environmental Impact
- Expansion of renewable energies on site through extension of the PV plant.
- Provision of short-term flexibility: integration of fluctuating renewable energy, and supply of system services in the form of aFRR (secondary control reserve).
- Sector coupling: integration of the WWTP in the energy market (hydrogen feed-in into the gas grid, hydrogen for public transport, gas storage and CHP management).
- Substitution of fossil fuels in different areas (gas and heat market, public transport and on-site of the WWTP).
- Coupling the regenerative energy generation on site with the advanced wastewater treatment to be implemented for the removal of OMP.
- Increasing resource efficiency by the use of the oxygen generated during electrolysis as the starting product for ozone production.
- Further reduction of OMP in the effluent of the WWTP by the innovative coupling of ozonation and GACF in combination (see Section 4.2) with resource-efficient operation of this process stage (see above) beyond the state of the art.
- Further reduction of phosphate and solid substances emissions into the Rhine River (see Section 5.1).
4. Planning Principles, Boundary Conditions and Results of the Feasibility Studies
4.1. Energetic Environment
4.2. Organic Micropollutant Elimination
- Increasing cleaning performance compared to one-step processes: in comparison to a pure ozone or GACF plant, both elimination mechanisms, oxidative degradation and GAC adsorption with their respective strengths are used for the removal of OMP. This effectively eliminates a wider range of micropollutants.
- Cleaning performance concerning micropollutant removal: depending on the desired elimination of various key substances, the ozone dosing in combination with the bed volume of the GAC can be adjusted.
- Reduction of the resource consumption for both stages and possibly the operating costs: Compared to single-stage ozonation or GAC processes, significantly lower ozone dosing and higher GAC bed volumes can be achieved. This reduces the resource consumption for each level.
4.2.1. Treated Amount of Wastewater
4.2.2. Plant Design
4.3. Electrolysis
4.3.1. Sustainable Operation Options
- The use of renewable surplus energy in the power grid/reaction to the curtailment of renewable energy systems,
- The use of a negative residual load caused by fluctuating renewable power,
- The structuring of fluctuating renewable energies for integration in renewable energy portfolios or for sales to consumers,
- Participation in the balancing power market/system services using renewable energy (in combination with points 1., 2. and 3.).
4.3.2. Operating Concept
4.3.3. Hydrogen and Oxygen Production
4.4. Interface O2 Storage
4.5. Energetic Situation after Implementation of the Concept
5. Environmental Effects
- Reduction of OMP discharges,
- Reduction of standard parameters discharges (total phosphorus (Ptot), total suspended solids (TSS) and chemical oxygen demand (COD)),
- Supporting the energy transition and,
- Multilevel reduction of CO2 emissions.
5.1. Environmental Improvements through Improved Water Protection
- Drugs,
- X-ray contrast agent,
- Household chemicals,
- Personal care and,
- Others.
5.2. Environmental Improvements through Contributions to the Energy Transition
- How can renewable power be stored at a large scale?
- How do we manage a heat supply system based on renewable energies?
- How can we realize a proper defossilization in the transport and industry sectors?
- How can we offer system services for the power grid based on RE?
- With the amount of hydrogen fed into the NGI at the WWTP, 250 apartments (each 50 m2 living space) could be supplied with renewable heat. In this case, the use of green hydrogen reduces greenhouse gas emissions by 83% compared to the use of fossil natural gas. Furthermore, hydrogen enables long-term storage of electricity.
- Supplementary to the feed into the NGI, the hydrogen produced at the WWTP can supply four fuel cell buses, saving around 190,000 L of diesel per year. By using green hydrogen in fuel cell buses, greenhouse gas emissions are reduced by 89% compared to a diesel bus.
- By offering secondary control reserve (aFRR), the electrolyzer contributes to the stability of the local power grid. Thus, conventional and CO2 producing power generation plants are replaced from the national balancing market.
- By expanding the PV capacity on the WWTP site, less biogas is required in the CHP unit and is saved for other situations. Thereby the overall greenhouse gas balance of the WWTP is improving.
5.3. Environmental Relief through CO2 Reductions
6. Techno-Economical Aspects
7. Discussion and Outlook
- Increasing the efficiency of the electrolyzer by using the co-products O2 (for ozonation) and heat (e.g., in the digester of WWTP);
- Economizing the purchase of liquid O2;
- Enhancing the energy transition by the expansion and integration of the local renewable energy generation in the energy system of the power-to-gas plants and by optimizing the overall energy management at the WWTP site.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA-QS | annual average-quality standard AA-QS, chronical quality standard |
| AC | active carbon |
| aFRR | automatic frequency restoration reserve—automatic activated reserve as system services for the power grid (secondary control) Background: the German transmission system operators (TSOs) have to maintain the balance between electricity generation and consumption within the control areas at all times. Therefore, the TSOs need different types of control reserve, which can be divided into three groups:
|
| CO2e | Equivalent carbon dioxide |
| COD | chemical oxygen demand |
| CQS | chronical quality standard or annual average-quality standard (AA-QS): “Chronic quality standards are recommended for water quality monitoring. They can be used for assessing pollution over an extended time period. For the continuous input of micropollutants from treated effluents, the chronic quality standard is particularly relevant and helps to protect the organisms against the consequences of long-term pollution.” [8] |
| DOC | dissolved organic carbon |
| ηel | electrical efficiency |
| GAC | granulated active carbon |
| GACF | granulated active carbon filter |
| OMP | organic micropollutant |
| MWel | Megawatt electrical power |
| NGI | natural gas infrastructure |
| PE | population equivalent |
| PE120 | PE120 is population equivalent, assuming 120 g of chemical oxygen demand (COD) per PE and per day |
| Ptot | total phosphorus |
| PtG | Power to Gas |
| PV | photovoltaic energy generation |
| Qd,h,max | Maximum hourly wastewater flow during dry weather |
| RE | renewable energies |
| TOC | total organic carbon |
| TSS | total suspended solids |
| TVM | Thermische Verwertung Mainz—sludge incineration plant |
| WWTP | wastewater treatment plant |
Appendix A. Oxygen and Ozone—Effects of different NO2-N concentrations
- DOC = 12.9 mg/L;
- Nitrite-Nitrogen (NO2-N) = 0.47 mg/L (25% percentile = 0.10 mg/L; 85% percentile = 1.00 mg/L).
- Reduction of NO2-N concentration in the feed by 50% to 0.235 mg/L;
- Reduction of NO2-N concentration in the feed by 100% to 0.0 mg/L.
- Maximum concentration: 0.4 gO3/gDOC;
- Average concentration: 0.2 gO3/gDOC.
| Status-Quo | −50% NO2-N | −100% NO2-N | Unit | ||
|---|---|---|---|---|---|
| necessary ozone dose | |||||
| feed to advanced treatment | maximum | 800.0 | 800.0 | 800.0 | L/s |
| average | 570.0 | 570.0 | 570.0 | L/s | |
| specific ozone dose with regard to DOC | maximum | 0.4 | 0.4 | 0.4 | g O3/g DOC |
| average | 0.2 | 0.2 | 0.2 | g O3/g DOC | |
| average feed concentration of DOC | 12.94 | 12.94 | 12.94 | mg DOC/L | |
| specific ozone dose with regard to nitrite-nitrogen | 3.43 | 3.43 | 3.43 | g O3/g N | |
| average feed concentration of nitrite | 0.47 | 0.24 | 0.00 | mg NO2-N/L | |
| necessary ozone dose | maximum | 6.79 | 5.98 | 5.18 | mg O3/L |
| average | 4.20 | 3.39 | 2.59 | mg O3/L | |
| Oxygen demand | |||||
| spec. oxygen demand for ozone production | 10.0 | 10.0 | 10.0 | mgO2/mgO3 | |
| necessary oxygen | maximum | 195.5 | 172.3 | 149.1 | kg O2/h |
| 4692 | 4135 | 3578 | kg O2/d | ||
| average | 86.2 | 69.7 | 53.1 | kg O2/h | |
| 2068.5 | 1671.5 | 1274.5 | kg O2/d | ||
| 754,989 | 610,098 | 465,206 | kg O2/a | ||
| Ozone demand | |||||
| Ozone demand | maximum | 19.55 | 17.23 | 14.91 | kg O3/h |
| average | 8.62 | 6.96 | 5.31 | kg O3/h |
| Change in Nitrite-Nitrogen Concentration | |||||||
|---|---|---|---|---|---|---|---|
| Status-Quo | −50% NO2-N | −100% NO2-N | Pos | ||||
| Oxygen demand | (kg/a) | 754,990 | 610,098 | 465,206 | P1 | ||
| Oxygen reduction compared to status-quo | (kg/a) | 0 | 144,892 | 289,783 | P2 | ||
| Use of oxygen from 20% base-load | Oxygen from electrolysis (20% base load) | (kg/a) | 292,000 | 292,000 | 292,000 | P3 | |
| Oxygen coverage gap | (kg/a) | 462,990 | 318,098 | 173,206 | P4 | =P1 − P3 | |
| Use of total oxygen (including aFRR-calls of the electrolyzer | Oxygen from electrolysis (from aFRR) | (kg/a) | 307,569 | 307,569 | 307,569 | P5 | |
| thereof need in ozonation | (kg/a) | 307,569 | 307,569 | 173,206 | P6 | =P5 (if P5 < P4) | |
| =P4 (if P5 > P4) | |||||||
| remaining oxygen coverage gap | (kg/a) | 155,421 | 10,529 | 0 | P7 | =P4 − P6 | |
| thereof remaining oxygen surplus | (kg/a) | 0 | 0 | 134,363 | P8 | =P5 − P6 | |
References
- United Nations. The Paris Agreement. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 22 April 2020).
- Quaschning, V. Klimaschutz oder Deichbau? Warum wir nicht erst 2038 aus der Kohleverstromung Aussteigen Können. Vortrag beim Energietag Rheinland-Pfalz 2019; Energietag Rheinland-Pfalz. TSB Transferstelle Bingen: Bingen, Germany, 2019. [Google Scholar]
- Sterner, M. Power-to-X and Sector Coupling: Setting the Right Legislative Framework for 230 Technology Pathways to Meet the Paris Climate Goals in Germany; IRES International Renewable Energy Storage: Düsseldorf, Germany, 2019. [Google Scholar]
- Jose, J.; Sandra Pinto, J.; Kotian, B.; Mathew Thomas, A.; Narayana Charyulu, R. Comparison of the regulatory outline of ecopharmacovigilance of pharmaceuticals in Europe, USA, Japan and Australia. Sci. Total Environ. 2020, 709, 134815. [Google Scholar] [CrossRef] [PubMed]
- Penru, Y.; Guillon, A.; Esperanza, M.; Martin, S.; Baig, S.; Miege, C.; Daval, A.; Cretollier, C.; Masson, M.; Coquery, M.; et al. Ozonation: Elimination des Micropolluants / Elimination von Mikroverunreinigungen durch Ozonung. Aqua Gas 2017, 97, 42–49. [Google Scholar]
- Peake, B.M.; Braund, R.; Tong, A.Y.C.; Tremblay, L.A. Regulatory Practices to Control the Discharge of Pharmaceuticals into the Environment. The Life-Cycle of Pharmaceuticals in the Environment; Elsevier: Amsterdam, The Netherlands, 2016; pp. 203–228. ISBN 9781907568251. [Google Scholar]
- Miarov, O.; Tal, A.; Avisar, D. A critical evaluation of comparative regulatory strategies for monitoring pharmaceuticals in recycled wastewater. J. Environ. Manag. 2020, 254, 109794. [Google Scholar] [CrossRef]
- oekotoxzentrum. Proposals for Acute and Chronic Quality Standards. Available online: https://www.ecotoxcentre.ch/expert-service/quality-standards/proposals-for-acute-and-chronic-quality-standards/?_ga=2.53865448.1586158361.1587446765-2105293851.1585640125 (accessed on 21 April 2020).
- Gerbersdorf, S.U.; Cimatoribus, C.; Class, H.; Engesser, K.-H.; Helbich, S.; Hollert, H.; Lange, C.; Kranert, M.; Metzger, J.; Nowak, W.; et al. Anthropogenic Trace Compounds (ATCs) in aquatic habitats—Research needs on sources, fate, detection and toxicity to ensure timely elimination strategies and risk management. Environ. Int. 2015, 79, 85–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EurEau. Treating Micropollutants at Waste Water Treatment Plants: Experiences and Developments from European Countries. Available online: http://www.eureau.org/resources/briefing-notes/3826-briefing-note-on-treating-micropollutants-at-the-wwtp/file (accessed on 13 May 2020).
- Antakyali, D.; Morgenschweis, K.; de Kort, T.; Herbst, H. Micropollutants in the Aquatic Environment and Their Removal in Wastewater Treatment Works: 9th European Waste Water Management Conference & Exhibition 12–13 October, Manchester Town Hall. Available online: https://www.researchgate.net/publication/312879251_MICROPOLLUTANTS_IN_THE_AQUATIC_ENVIRONMENT_AND_THEIR_REMOVAL_IN_WASTEWATER_TREATMENT_WORKS (accessed on 17 May 2020).
- Metz, F. (Ed.) From Network Structure to Policy Design in Water Protection. A Comparative Perspective on Micropollutants in the Rhine River Riparian Countries; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-55693-2. [Google Scholar]
- Siegrist, H.; Joss, A.; Boehler, M.; McArdell, C.S.; Ternes, T. Organic micropollutant control. In Advances in Wastewater Treatment; Mannina, G., Ekama, G., Ødegaard, H., Olsson, G., Eds.; IWA Publishing: London, UK, 2019. [Google Scholar]
- BMU/UBA. Ergebnispapier—Ergebnisse der Phase 2 des Stakeholder-Dialogs »Spurenstoffstrategie des Bundes« zur Umsetzung von Maßnahmen für die Reduktion von Spurenstoffeinträgen in die Gewässer. 2019. Available online: https://www.bmu.de/download/ergebnisse-der-phase-2-des-stakeholder-dialogs-spurenstoffstrategie-des-bundes/ (accessed on 9 July 2020).
- VSA. Projekte mit Ozon. VSA-Plattform “Verfahrenstechnik Mikroverunreinigungen”. 2019. Available online: https://www.micropoll.ch/anlagen-projekte/ozon/ (accessed on 28 February 2019).
- IRENA. Renewable Capacity Statistics 2020. Available online: https://www.irena.org/publications/2020/Mar/Renewable-Capacity-Statistics-2020 (accessed on 6 April 2020).
- IRENA. Renewable Capacity Highlights. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Mar/IRENA_RE_Capacity_Highlights_2020.pdf?la=en&hash=B6BDF8C3306D271327729B9F9C9AF5F1274FE30B (accessed on 31 March 2020).
- Sterner, M.; Stadler, I. Handbook of Energy Storage. Demand, Technologies, Integration; Springer Berlin: Berlin, Germany, 2018; ISBN 978-3-662-55504-0. [Google Scholar]
- Schäfer, M.; Gretzschel, O.; Steinmetz, H. The Possible Roles of Wastewater Treatment Plants in Sector Coupling. Energies 2020, 13, 2088. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, M. Short-term flexibility for energy grids provided by wastewater treatment plants with anaerobic sludge digestion. Water Sci. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.G.; Gretzschel, O.; Schäfer, M.; Hüesker, F.; Salomon, D.; Bidlingmaier, A.; Zdrallek, M.; Kornrumpf, T.; Simon, R.; Hanke, B.; et al. Abwasserreinigungsanlagen als Regelbaustein in intelligenten Verteilnetzen mit Erneuerbarer Energieerzeugung—Arrivee. Schlussbericht Arrivee. 2017. Available online: http://edok01.tib.uni-hannover.de/edoks/e01fb17/899010695.pdf (accessed on 13 December 2017).
- Steinmetz, H.; Schmitt, T.G.; Schäfer, M.; Gretzschel, O.; Krieger, S.; Alt, K.; Zydorczyk, S.; Bender, V.; Pick, E. Konzeptstudie—Klimafreundliche und ressourceneffiziente Anwendung der Wasserelektrolyse zur Erzeugung von regenerativen Speichergasen kombiniert mit einer weitergehenden Abwasserbehandlung zur Mikroschadstoffelimination auf Kläranlagen—Arrived. Studie im Auftrag der Wirtschaftsbetriebe Mainz (Unpublished); Final Report; Tectraa an der TU Kaiserslautern: Kaiserslautern, Germany, 2018. [Google Scholar]
- Rößler, A.; Rau, W.; Metzger, S. Vorkommen von Spurenstoffen in Kläranlagenzuläufen in Baden-Württemberg. Wasser und Abfall 2018, 40–49. Available online: https://koms-bw.de/cms/content/media/Vorkommen%20von%20Spurenstoffen%20in%20Klaeranlagenzulaeufen%20in%20BW.pdf (accessed on 9 July 2020).
- Schmitt, T.G.; Knerr, H.; Gretzschel, O.; Kolisch, G.; Taudien, Y. Relevanz, Möglichkeiten und Kosten einer Elimination von Mikroschadstoffen auf kommunalen Kläranlagen in Rheinland-Pfalz, aufgezeigt am Beispiel der Nahe— Mikro_N. Studie im Auftrag des Ministeriums für Umwelt, Landwirtschaft, Ernährung, Weinbau und Forsten (MULEWF) des Landes Rhein-land-Pfalz, Deutschland. 2016. Available online: https://wasser.rlp-umwelt.de/servlet/is/1130/ (accessed on 21 August 2018).
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef]
- Zhang, Y.; Geissen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Umweltbundesamt. ETOX: Informationssystem Ökotoxikologie und Umweltqualitätsziele. Available online: https://webetox.uba.de/webETOX/public/search/ziel/open.do (accessed on 11 September 2018).
- ICSR/IKSR. Micropollutants in the Rhine Catchment Area/Mikroverunreinigungen im Rheineinzugsgebiet Bilanz 2017: Bericht Nr. International Commission for the Protection of the Rhine (ICPR). Available online: https://www.iksr.org/en/public-relations/documents/archive/technical-reports/reports-and-brochures-individual-presentation?tx_news_pi1%5Baction%5D=detail&tx_news_pi1%5Bcontroller%5D=News&tx_news_pi1%5Bnews%5D=527&cHash=7ec1cb6e82a0271e9bf7caa6b7dab3f8 (accessed on 10 April 2018).
- Angerbauer, F. Spurenstoffmessung im Rhein an der Messstelle Mainz—Ergebnisse 2010. Personal communication via E-Mail, 2019. [Google Scholar]
- DWA (Ed.) Energiecheck und Energieanalyse. Instrumente zur Energieoptimierung von Abwasseranlagen; DWA: Hennef, Germany, 2015; ISBN 978-3-88721-276-6. [Google Scholar]
- DWA. Anthropogene Spurenstoffe, Krankheitserreger und Antibiotikaresistenzen im Wasserkreislauf. 2017. Available online: https://de.dwa.de/files/_media/content/01_DIE%20DWA/Forschung%20und%20Innovation/Anthropogene_Spurenstoffe_Krankheitserreger_TransRisk_Druckfassung_komprimiert-20170130.pdf (accessed on 21 February 2019).
- Böhler, M.; Blunschi, M.; Czekalski, N.; Fleiner, J.; Kienle, C.; Langer, M.; McArdell, C.S.; Teichler, R.; Siegrist, H. Biologische Nachbehandlung von kommunalem Abwasser nach Ozonung—ReTREAT. Abschlussbericht für das Bundesamt für Umwelt (Bafu) im Rahmen eines Projektes der Technologieförderung. 2017. Available online: http://www.oekotoxzentrum.ch/media/182953/2017_boehler_retreat_schlussbericht.pdf (accessed on 9 July 2020).
- Östman, M.; Björlenius, B.; Fick, J.; Tysklind, M. Effect of full-scale ozonation and pilot-scale granular activated carbon on the removal of biocides, antimycotics and antibiotics in a sewage treatment plant. Sci. Total Environ. 2019, 649, 1117–1123. [Google Scholar] [CrossRef]
- Rödel, S.; Günthert, W.; Christ, O.; Miller, E.; Schatz, R.; Zech, T.; Bleisteiner, S.; Eßlinger, M.; Sengl, M.; Rehbein, V.; et al. Erfahrungen und Ergebnisse aus dem Betrieb der vierten Reinigungsstufe in Weißenburg: Teil 1: Untersuchungsprogramm und Ergebnisse. KA Korrespondenz Abwasser Abfall 2019, 66, 200–209. [Google Scholar]
- Baresel, C.; Ek, M.; Ejhed, H.; Allard, A.-S.; Magnér, J.; Dahlgren, L.; Westling, K.; Wahlberg, C.; Fortkamp, U.; Söhr, S.; et al. Sustainable treatment systems for removal of pharmaceutical residues and other priority persistent substances. Water Sci. Technol. 2019, 79, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, H.; Schmitt, T.G.; Schäfer, M.; Gretzschel, O.; Krieger, S.; Alt, K.; Zydorczyk, S.; Bender, V.; Pick, E. Ergänzende Betrachtungen zur Konzeptstudie „arrived“ Vorzugsvariante-. Studie im Auftrag der Wirtschaftsbetriebe Mainz (Unpublished); Final Report; Tectraa an der TU Kaiserslautern: Kaiserslautern, Germany, 2019. [Google Scholar]
- Müller-Syring, G.; Henel, M.; Köppel, W.; Sterner, M.; Höcher, T. Entwicklung von modularen Konzepten zur Erzeugung, Speicherung und Einspeisung von Wasserstoff und Methan ins Erdgasnetz. Abschlussbericht—DVGW-Förderzeichen G 1-07-10; DVGW Forschung: 2013. Available online: https://www.dvgw.de/medien/dvgw/forschung/berichte/g1_07_10.pdf (accessed on 9 July 2020).
- Ateş, A. Cost of Reducing Micropollutant Load from Urban Wastewater Treatment Plants in Turkey. 2019. Available online: http://etd.lib.metu.edu.tr/upload/12625042/index.pdf (accessed on 17 May 2020).
- Austermann-Haun, U.; Meier, J.F.; Nahrstedt, A.; Sikorski, D.; Kuhlmann, S.; Alt, K. Spurenstoffelimination auf der Kläranlage Detmold mittels der Kombination von Ozon mit nachgeschalteter Aktivkohlefiltration. Abschlussbericht, gerichtet an das Ministerium für Klimaschutz, Umwelt, Landwirtschaft, Natur- und Verbraucherschutz des Landes Nordrhein-Westfalen. 2017. Available online: https://www.lanuv.nrw.de/fileadmin/forschung/170815_Pilotvorhaben%20Detmold/O-01-12-DT-Spurenstoffelimination-KA-DT_Abschlussbericht.pdf (accessed on 12 October 2018).
- Gretzschel, O.; Schäfer, M.; Steinmetz, H.; Schmitt, T.G. Combined Implementation of Power-to-Gas and Elimination of Micropollutants—A Feasibility Study for WWTP Mainz. In Proceedings of the 13th International Renewable Energy Storage Conference (IRES 2019), Düsseldorf, Germany, 12–14 March 2019. [Google Scholar]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Huneke, F.; Linkenheil, C.P.; Niggemeier, M.-L. Kalte Dunkelflaute: Robustheit des Stromsystems bei Extremwetter. Available online: https://www.energybrainpool.com/fileadmin/download/Studien/Studie_2017-06-26_GPE_Studie_Kalte-Dunkelflaute_Energy-Brainpool.pdf (accessed on 4 May 2020).
- Sterner, M.; Stadler, I. Energiespeicher-Bedarf, Technologien, Integration, Auflage; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- h2agentur. Wasserstoff zur Speicherung Erneuerbarer Energie: PEM-Elektrolyseure. Available online: https://h2agentur.de/elektrolyseure/ (accessed on 11 May 2020).
- h2agentur. Pressure of the Oxygen Generated by PEM Electrolysis, Personal Confirmation by Phone from h2agentur to Stefan Krieger Hydroingenieure, Kaiserslautern, Germany: 2019.
- Tjarks, G. PEM-Elektrolyse-Systeme zur Anwendung in Power-to-Gas Anlagen; Forschungszentrum Jülich GmbH, Zentralbibliothek: Jülich, Germany, 2017; ISBN 978-3-95806-217-7. [Google Scholar]
- Mousel, D.; Palmowski, L.; Pinnekamp, J. Energy demand for elimination of organic micropollutants in municipal wastewater treatment plants. Sci. Total Environ. 2017, 575, 1139–1149. [Google Scholar] [CrossRef]
- Pinnekamp, J.; Bolle, F.-W.; Palmowski, L.; Veltmann, K.; Mousel, D.; Mauer, C.; Eckers, S. Energiebedarf von Verfahren zur Elimination von organischen Spurenstoffen. Abschlussbericht zum gleichnamigen Forschungsprojekt, gefördert durch das Ministerium für Klimaschutz, Umwelt, Landwirtschaft, Natur- und Verbraucherschutz des Landes Nordrhein-Westfalen (MKULNV). 2011. Available online: http://www.lanuv.nrw.de/wasser/abwasser/forschung/pdf/Abschlussbericht_ENVELOS.pdf (accessed on 28 September 2012).
- Hillenbrand, T.; Tettenborn, F.; Fuchs, S.; Tochovski, S.; Metzger, S.; Tjoeng, I.; Wermter, P.; Kersting, M.; Hecht, D.; WErbeck, N.; et al. Maßnahmen zur Verminderung des Eintrages von Mikroschadstoffen in die Gewässer—Phase 2. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/377/publikationen/mikroschadstoffen_in_die_gewasser-phase_2.pdf (accessed on 9 July 2020).
- Altmann, J.; Rehfeld, D.; Träder, K.; Sperlich, A.; Jekel, M. Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef]
- Hamoda, M.F.; Al-Ghusain, I.; Al-Mutairi, N.Z. Sand filtration of wastewater for tertiary treatment and water reuse. Desalination 2004, 164, 203–211. [Google Scholar] [CrossRef]
- Lardon, L.; Thorberg, D.; Krosgaard, L. Biogas valorization and efficient energy management—Technical and economic analysis of biological methanation. POWERSTEP Deliverable D 3. In Powerstep: Your Flush, Our Energy; Kompetenzzentrum, W.B., Ed.; Kompetenzzentrum Wasser Berlin: Berlin, Germany, 2018; Available online: http://powerstep.eu/system/files/generated/files/resource/d3-2-technical-and-economic-analysis-of-biological-methanationdeliverable.pdf (accessed on 16 March 2020).
- Juhrich, K. CO2-Emissionsfaktoren für Fossile Brennstoffe. Available online: https://www.umweltbundesamt.de/publikationen/co2-emissionsfaktoren-fuer-fossile-brennstoffe (accessed on 14 January 2019).
- Koch, F. E-Busse mit Brennstoffzelle—Europäische Markteinführungsinitiative; EnergieAgentur NRW: Aachen, Germany, 2016. [Google Scholar]
- Eichhammer, W.; Fleiter, T.; Schlomann, B. Energieverbrauch und CO2-Emissionen industrieller Prozesstechnologien. Einsparpotenziale, Hemmnisse und Instrumente; Fraunhofer-Verl.: Stuttgart, Germany, 2013; Available online: https://www.isi.fraunhofer.de/content/dam/isi/dokumente/ccx/2013/Umweltforschungsplan_FKZ-370946130.pdf (accessed on 9 July 2020).
- UBA. ProBas-Prozessorientierte Basisdaten für Umweltmanagementsysteme. Available online: http://www.probas.umweltbundesamt.de/php/index.php (accessed on 17 December 2018).
- Schutzgemeinschaft Deutscher Wald, S.D.W. Was leistet der Wald für uns? Available online: https://www.sdw.de/waldwissen/oekosystem-wald/waldleistungen/index.html (accessed on 4 May 2020).
- Huneke, F. Auf dem Weg in die Wettbewerbsfähigkeit: Elektrolysegase erneuerbaren Ursprungs. 2018. Available online: https://www.energybrainpool.com/fileadmin/download/Studien/Kurzanalyse_2018-03-19_GPE_Kurzanalyse_Kostenentwicklung-von-Elektrolysegas-erneuerbaren-Ursprungs.pdf (accessed on 5 May 2020).
- Greenpeace Energy. Unser Windgas im Detail. Available online: https://www.greenpeace-energy.de/privatkunden/oekogas/unser-windgas-im-detail.html (accessed on 18 May 2020).
- Parra, D.; Zhang, X.; Bauer, C.; Patel, M.K. An integrated techno-economic and life cycle environmental assessment of power-to-gas systems. Appl. Energy 2017, 193, 440–454. [Google Scholar] [CrossRef]
- Blanco, H.; Faaij, A. A review at the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Eveloy, V.; Gebreegziabher, T. A Review of Projected Power-to-Gas Deployment Scenarios. Energies 2018, 11, 1824. [Google Scholar] [CrossRef] [Green Version]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Erneuerbare-Energien-Gesetz—EEG 2017/Renewable Energy Sources Act. (EEG 2017). EEG. 2020. Available online: https://www.bmwi.de/Redaktion/DE/Downloads/E/eeg-2017-gesetz-en.pdf?__blob=publicationFile&v=8 (accessed on 19 May 2020).
- BDEW. BDEW-Strompreisanalyse 2020. Available online: https://www.bdew.de/energie/strom-und-gaspreisanalysen/ (accessed on 18 May 2020).
- 50Hertz Transmission GmbH, Amprion GmbH, TransnetBW GmbH, Tennet TSO GmbH. EEG-Umlage. Available online: https://www.netztransparenz.de/EEG/EEG-Umlagen-Uebersicht (accessed on 19 May 2020).
- regelleistung.net. Balancing Market in Germany. Available online: https://www.regelleistung.net/ext/static/market-information?lang=en (accessed on 29 June 2020).
- Bui, X.T.; Vo, T.P.T.; Ngo, H.H.; Guo, W.S.; Nguyen, T.T. Multicriteria assessment of advanced treatment technologies for micropollutants removal at large-scale applications. Sci. Total Environ. 2016, 563–564, 1050–1067. [Google Scholar] [CrossRef] [PubMed]
- Alt, K. Umnutzung vorhandener Flockungsfiltrationsanlagen zu Aktivkohle- und Ozonanlagen; 50 Jahre Oswald-Schulze Stiftung; Technische Innovationen in der Abwasserreinigung: Münster, Germany, 2018. [Google Scholar]







 |
| Characteristics | Amount | Unit |
|---|---|---|
| Power | 1.25 | MW |
| Hours of operation 20% band | 8585 | h/a |
| Full load hours | 3500 | h/a |
| O2 production (20% band) | 292,000 | kg/a |
| O2 production (aFRR) | 307,569 | kg/a |
| Total O2 production | 599,569 | kg/a |
| H2 production | 2975 | MWh/a |
| Amount | Unit | |
|---|---|---|
| specific oxygen demand for ozone production | 10.0 | mgO2/mgO3 |
| maximum required amount of oxygen | 149.1 | kg O2/h |
| average required amount of oxygen | 53.1 | kg O2/h |
 |
| Substance | Substance Group/Field of Application | Specification | Elimination by Advanced Treatment [%] 1 | Average Effluent Concentration n = 5 [µg/L] | Dry Weather Runoff 2017 [Mio m3/a] | Calculated Emission Load Status Quo (kg/a) | Reduction of Emission Load in (kg/a) with 4th Treatment Stage | CQS ([µg/L) 4 |
|---|---|---|---|---|---|---|---|---|
| Carbamazepine | drug | anti-epileptic | 90 | 0.45 | 15.59 | 7.04 | 6.3 | 2.00 |
| Diclofenac | anti-inflammatory | 90 | 2.34 | 15.59 | 36.47 | 32.8 | 0.05 | |
| Ibuprofen | anti-inflammatory | 80 | 0.22 | 15.59 | 3.42 | 2.7 | 0.11 | |
| Metoprolol | beta blockers | 50 | 1.71 | 15.59 | 26.69 | 13.3 | 8.60 | |
| Sulfamethoxazole | antibiotics | 90 | 0.29 | 15.59 | 4.46 | 4.0 | 0.60 | |
| Amidotrizoic acid | X-ray contrast agent | X-ray contrast agent | 10 | 2.76 | 15.59 | 43.02 | 4.3 | - 5 |
| Iomeprol | X-ray contrast agent | 50 | 75.80 | 15.59 | 1,181.41 | 590.7 | - | |
| Perfluoroctane sulfonic acid (PFOS) | household chemicals | fluorosurfactant | 50 | 0.046 2 | 15.59 | 0.72 | 0.7 | 0.022 |
| Perfluorooctance acid (PFOA) | fluorosurfactant | 50 | 0.010 2 | 15.59 | 0.14 | 0.1 | - | |
| Terbutryn | herbicide | 80 | 0.044 2 | 15.59 | 0.69 | 0.5 | 0.065 | |
| HHCB | personal care | synthetic fragrances | 90 | 0.54 | 15.59 | 8.48 | 7.6 | - |
| Triclosan | disinfectant | 80 | 0.046 3 | 15.59 | 0.72 | 0.5 | 0.11 | |
| EDTA | others | complexing agent | 10 | 96.00 | 15.59 | 1496.24 | 149.6 | 2200 |
| Oxygen | Unit | Avoided Costs for Liquid O2 1 | Unit | |
|---|---|---|---|---|
| Oxygen demand for ozone production: | 465,206 | kgO2/a | ||
| Oxygen production by 20%-load of electrolyzer: | 292,000 | kgO2/a | 29,200 | €/a |
| Resulting coverage gap: | 173,206 | kgO2/a | ||
| Oxygen production by control energy calls (aFRR): | 307,569 | kgO2/a | ||
| Usable to close coverage gap: | 173,206 | kgO2/a | 17,320 | €/a |
| Costs | Unit | |
|---|---|---|
| O2 dryer | 180,000–264,000 | € |
| Compressor | 80,000–131,000 | € |
| O2 storage (100 m3) | 170,000–220,000 | € |
| Pressure reduction | 66,000 | € |
| Set gross costs | (496,000)–681,000 | € |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gretzschel, O.; Schäfer, M.; Steinmetz, H.; Pick, E.; Kanitz, K.; Krieger, S. Advanced Wastewater Treatment to Eliminate Organic Micropollutants in Wastewater Treatment Plants in Combination with Energy-Efficient Electrolysis at WWTP Mainz. Energies 2020, 13, 3599. https://doi.org/10.3390/en13143599
Gretzschel O, Schäfer M, Steinmetz H, Pick E, Kanitz K, Krieger S. Advanced Wastewater Treatment to Eliminate Organic Micropollutants in Wastewater Treatment Plants in Combination with Energy-Efficient Electrolysis at WWTP Mainz. Energies. 2020; 13(14):3599. https://doi.org/10.3390/en13143599
Chicago/Turabian StyleGretzschel, Oliver, Michael Schäfer, Heidrun Steinmetz, Erich Pick, Kim Kanitz, and Stefan Krieger. 2020. "Advanced Wastewater Treatment to Eliminate Organic Micropollutants in Wastewater Treatment Plants in Combination with Energy-Efficient Electrolysis at WWTP Mainz" Energies 13, no. 14: 3599. https://doi.org/10.3390/en13143599