Hydrothermal Desorption of Cs with Oxalic Acid from Hydrobiotite and Wastewater Treatment by Chemical Precipitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
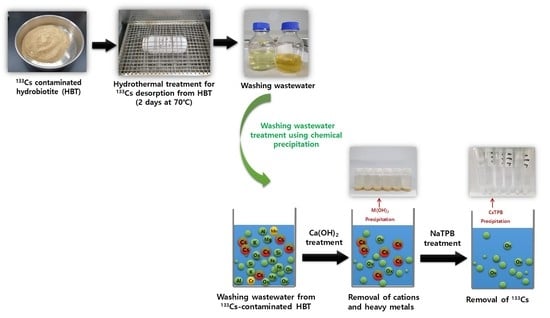
2.2. Adsorption and Hydrothermal Desorption of Non-Radioactive 133Cs–HBT
2.3. Adsorption of Radioactive 137Cs to HBT and its Desorption by Oxalic Acid
2.4. Characteristics of Cs-Desorbed Soil
2.5. Washing Wastewater Treatment Using Chemical Precipitation
3. Results and Discussion
3.1. Cs Desorption by Oxalic Acid from HBT Contaminated with 133Cs and 137Cs
3.2. The Release of Al, Fe, Mg, and Si from 133Cs–HBT by Oxalic Acid
3.3. Structural and Morphological Effects of Oxalic Acid Treatment on 133Cs–HBT
3.4. Removal of Cations from Washing Wastewater of 133Cs–HBT
3.5. Removal of Cs from Washing Wastewater of 133Cs–HBT
3.6. Application of Washing Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.; Park, S.-M.; Jeon, E.-K.; Baek, K. Selective and irreversible adsorption mechanism of cesium on illite. Appl. Geochem. 2017, 85, 188–193. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Wang, L.; Fan, Q.; Pan, D.; Li, J.; Chi, F.; Xie, Y.; Yu, S.; Xiao, C. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci. China Chem. 2019, 62, 933–967. [Google Scholar] [CrossRef]
- Rich, C.; Black, W. Pottasium exchange as affected by cation size, pH, and mineral structure. Soil Sci. 1964, 97, 384–390. [Google Scholar] [CrossRef]
- Brouwer, E.; Baeyens, B.; Maes, A.; Cremers, A. Cesium and rubidium ion equilibriums in illite clay. J. Phys. Chem. 1983, 87, 1213–1219. [Google Scholar] [CrossRef]
- Boettcher, A. Vermiculite, hydrobiotite, and biotite in the Rainy Creek igneous complex near Libby, Montana. Clay Miner. 1966, 6, 283–296. [Google Scholar] [CrossRef]
- Brindley, G.; Zalba, P.E.; Bethke, C.M. Hydrobiotite, a regular 1: 1 interstratification of biotite and vermiculite layers. Am. Mineral. 1983, 68, 420–425. [Google Scholar]
- Yamada, H.; Yokoyama, S.; Watanabe, Y.; Suzuki, M.; Suzuki, S.; Hatta, T. Cesium-adsorption Behavior of Weathered Biotite from Fukushima Prefecture Depends on the Degree of Vermiculitization. J. Ion. Exch. 2014, 25, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Nakao, A.; Thiry, Y.; Funakawa, S.; Kosaki, T. Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand. Soil Sci. Plant Nutr. 2008, 54, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Mukai, H.; Motai, S.; Yaita, T.; Kogure, T. Identification of the actual cesium-adsorbing materials in the contaminated Fukushima soil. Appl. Clay Sci. 2016, 121, 188–193. [Google Scholar] [CrossRef]
- Dzene, L.; Tertre, E.; Hubert, F.; Ferrage, E. Nature of the sites involved in the process of cesium desorption from vermiculite. J. Colloid Interface Sci. 2015, 455, 254–260. [Google Scholar] [CrossRef]
- Mukai, H.; Hirose, A.; Motai, S.; Kikuchi, R.; Tanoi, K.; Nakanishi, T.M.; Yaita, T.; Kogure, T. Cesium adsorption/desorption behavior of clay minerals considering actual contamination conditions in Fukushima. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.H. Principles of Soil Chemistry; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sanjay, G.; Sugunan, S. Acid activated montmorillonite: An efficient immobilization support for improving reusability, storage stability and operational stability of enzymes. J. Porous Mater. 2008, 15, 359–367. [Google Scholar] [CrossRef]
- Berner, R.A.; Holdren Jr, G.R. Mechanism of feldspar weathering—II. Observations of feldspars from soils. Geochim. Et Cosmochim. Acta 1979, 43, 1173–1186. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, C.W.; Yang, H.-M.; Seo, B.-K.; Lee, B.-S.; Lee, K.-W.; Park, S.J. Comparison of Cs desorption from hydrobiotite by cationic polyelectrolyte and cationic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 382–388. [Google Scholar] [CrossRef]
- Jolin, W.C.; Kaminski, M.J.C. Sorbent materials for rapid remediation of wash water during radiological event relief. Chemosphere 2016, 162, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaheri, A.; Moheb, A.; Keshtkar, A.; Shirani, A. Uranium separation from wastewater by electrodialysis. J. Environ. Health Sci. Eng. 2010, 7, 423–430. [Google Scholar]
- Chmielewski, A.; Harasimowicz, M.; Zakrzewska-Trznadel, G. Membrane technologies for liquid radioactive waste treatment. Czechoslov. J. Phys. 1999, 49, 979–985. [Google Scholar] [CrossRef]
- Ku, Y.; Jung, I.-L. Photocatalytic reduction of Cr (VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res. 2001, 35, 135–142. [Google Scholar] [CrossRef]
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 2006, 83, 106–113. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, B.H.; Yang, H.-M.; Seo, B.-K.; Lee, K.-W. Enhanced desorption of Cs from clays by a polymeric cation-exchange agent. J. Hazard. Mater. 2017, 327, 127–134. [Google Scholar] [CrossRef]
- Komadel, P.; Madejová, J. Acid activation of clay minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 385–409. [Google Scholar]
- Krupskaya, V.; Zakusin, S.; Tyupina, E.; Dorzhieva, O.; Zhukhlistov, A.; Belousov, P.; Timofeeva, M. Experimental study of montmorillonite structure and transformation of its properties under treatment with inorganic acid solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Al-Essa, K. Activation of Jordanian bentonite by hydrochloric acid and its potential for olive mill wastewater enhanced treatment. J. Chem. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Malmström, M.; Banwart, S.; Lewenhagen, J.; Duro, L.; Bruno, J. The dissolution of biotite and chlorite at 25 C in the near-neutral pH region. J. Contam. Hydrol. 1996, 21, 201–213. [Google Scholar] [CrossRef]
- Malmström, M.; Banwart, S. Biotite dissolution at 25 C: The pH dependence of dissolution rate and stoichiometry. Geochim. Et Cosmochim. Acta 1997, 61, 2779–2799. [Google Scholar] [CrossRef]
- Liu, C.; Zachara, J.M.; Smith, S.C.; McKinley, J.P.; Ainsworth, C.C. Desorption kinetics of radiocesium from subsurface sediments at Hanford Site, USA. Geochim. Et Cosmochim. Acta 2003, 67, 2893–2912. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.-N.; Choi, W.-K.; Jung, C.-H.; Moon, J.-K. Development of a washing system for soil contaminated with radionuclides around TRIGA reactors. J. Ind. Eng. Chem. 2007, 13, 406–413. [Google Scholar]
- Wendling, L.A.; Harsh, J.B.; Palmer, C.D.; Hamilton, M.A.; Flury, M. Cesium sorption to illite as affected by oxalate. Clays Clay Miner. 2004, 52, 375–381. [Google Scholar] [CrossRef]
- Sawhney, B. Potassium and cesium ion selectivity in relation to clay mineral structure. Clays Clay Miner. 1970, 18, 47–52. [Google Scholar] [CrossRef]
- Sawhney, B. Selective sorption and fixation of cations by clay minerals: A review. Clays Clay Miner. 1972, 20, 93–100. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.; Kang, H.-D.; Kim, W.; Doh, S.-H.; Kim, D.-S.; Kim, B.-K. The accumulation of radiocesium in coarse marine sediment: Effects of mineralogy and organic matter. Mar. Pollut. Bull. 2007, 54, 1341–1350. [Google Scholar] [CrossRef]
- Xavier, K.C.M.; Santos, M.d.S.F.d.; Santos, M.R.M.C.; Oliveira, M.E.R.; Carvalho, M.W.N.C.; Osajima, J.A.; Silva Filho, E.C.d. Effects of acid treatment on the clay palygorskite: XRD, surface area, morphological and chemical composition. Mater. Res. 2014, 17, 3-08. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Hu, H.; Zhang, T.; Zhou, Y. Dissolution of kaolinite induced by citric, oxalic, and malic acids. J. Colloid Interface Sci. 2005, 290, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Fein, J.B.; Hestrin, J.E. Experimental studies of oxalate complexation at 80 C: Gibbsite, amorphous silica, and quartz solubilities in oxalate-bearing fluids. Geochim. Et Cosmochim. Acta 1994, 58, 4817–4829. [Google Scholar] [CrossRef]
- Welch, S.A.; Ullman, W.J. The temperature dependence of bytownite feldspar dissolution in neutral aqueous solutions of inorganic and organic ligands at low temperature (5–35 C). Chem. Geol. 2000, 167, 337–354. [Google Scholar] [CrossRef]
- Surdam, R.C.; Boese, S.W.; Crossey, L.J. The chemistry of secondary porosity: Part 2. Aspects of porosity modification. Calstic Diagenesis 1984, 127–149. Available online: archives.datapages.com/data/specpubs/sandsto2/data/a059/0001/0100/0127.htm (accessed on 13 May 2020).
- Huang, W.; Kiang, W. Laboratory dissolution of plagioclase feldspars in water and organic acids at room temperature. Am. Mineral. J. Earth Planet. Mater. 1972, 57, 1849–1859. [Google Scholar]
- Mukai, H.; Tamura, K.; Kikuchi, R.; Takahashi, Y.; Yaita, T.; Kogure, T. Cesium desorption behavior of weathered biotite in Fukushima considering the actual radioactive contamination level of soils. J. Environ. Radioact. 2018, 190, 81–88. [Google Scholar] [CrossRef]
- Murakami, T.; Utsunomiya, S.; Yokoyama, T.; Kasama, T. Biotite dissolution processes and mechanisms in the laboratory and in nature: Early stage weathering environment and vermiculitization. Am Mineral. 2003, 88, 377–386. [Google Scholar] [CrossRef]
- Turpault, M.-P.; Trotignon, L. The dissolution of biotite single crystals in dilute HNO3 at 24 C: Evidence of an anisotropic corrosion process of micas in acidic solutions. Geochim. Et Cosmochim. Acta. 1994, 58, 2761–2775. [Google Scholar] [CrossRef]
- Kalinowski, B.E.; Schweda, P. Kinetics of muscovite, phlogopite, and biotite dissolution and alteration at pH 1–4, room temperature. Geochim. Et Cosmochim. Acta. 1996, 60, 367–385. [Google Scholar] [CrossRef]
- Murakami, T.; Ito, J.-I.; Utsunomiya, S.; Kasama, T.; Kozai, N.; Ohnuki, T. Anoxic dissolution processes of biotite: Implications for Fe behavior during Archean weathering. Earth Planet. Sci. Lett. 2004, 224, 117–129. [Google Scholar] [CrossRef]
- Zhong, S.; Sun, S.; Chen, T.; Peng, S.J. Influence of hydrochloric acid-dissolution on the structure of montmorillonite. J. Chi. Ceram. Soc. 2006, 34, 1162. [Google Scholar]
- Wang, L.K.; Vaccari, D.A.; Li, Y.; Shammas, N.K. Chemical precipitation. In Physicochemical Treatment Processes; Springer: Berlin, Germany, 2005; pp. 141–197. [Google Scholar]
- Chen, B.; Qu, R.; Shi, J.; Li, D.; Wei, Z.; Yang, X.; Wang, Z. Heavy metal and phosphorus removal from waters by optimizing use of calcium hydroxide and risk assessment. Environ. Pollut. 2012, 1, 38. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Rüdorff, W.; Zannier, H. Argentometrie von Kalium und organischen N-haltigen Basen mit Natrium-tetraphenyloborat. Angew. Chem. 1954, 66, 638–639. [Google Scholar] [CrossRef]
- Amin, A.-A.M. Determination of Ammonium, Potassium, Rubidium, or Cesium via Cationic Resin Exchange of Their Tetraphenylboron Salts. Chem. Anal 1956, 45, 65. [Google Scholar]
- Ponder, S.M.; Mallouk, T.E. Recovery of ammonium and cesium ions from aqueous waste streams by sodium tetraphenylborate. Ind. Eng. Chem. Res. 1999, 38, 4007–4010. [Google Scholar] [CrossRef]
- Lee, E.-H.; Lim, J.-G.; Chung, D.-Y.; Yang, H.-B.; Kim, K.-W. Selective removal of Cs and Re by precipitation in a Na2CO3–H2O2 solution. J. Radioanal. Nucl. Chem. 2010, 284, 387–395. [Google Scholar] [CrossRef]
- Rogers, H.; Bowers, J.; Gates-Anderson, D. An isotope dilution–precipitation process for removing radioactive cesium from wastewater. J. Hazard. Mater. 2012, 243, 124–129. [Google Scholar] [CrossRef]
- Soliman, M.A.; Rashad, G.M.; Mahmoud, M.R. Fast and efficient cesium removal from simulated radioactive liquid waste by an isotope dilution–precipitate flotation process. Chem. Eng. J. 2015, 275, 342–350. [Google Scholar] [CrossRef]
- Council, N.R. Alternatives for High-Level Waste Salt Processing at the Savannah River Site; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Gupta, A.K.; Hanrahan, R.J.; Walker, D.D. Radiolysis of sodium and potassium tetraphenylborate in aqueous systems. J. Phys. Chem. 1991, 95, 3590–3594. [Google Scholar] [CrossRef]
- Kim, G.-N.; Kim, S.-S.; Choi, J.-W. Development of an agent suited for adsorbing Cs-137 from ash and soil waste solutions. Sep. Purif. Technol. 2017, 173, 193–199. [Google Scholar] [CrossRef]










| Clay | ConcenTration (mol/L) | Reaction Time | MgO | Al2O3 | SiO2 | K2O | TiO2 | Fe2O3 |
|---|---|---|---|---|---|---|---|---|
| HBT | Raw-HBT | D.W. | 25.53 ± 0.03 | 11.81 ± 0.02 | 43.44 ± 0.05 | 6.30 ± 0.25 | 1.18 ± 0.01 | 8.05 ± 0.02 |
| Oxalic acid 1 M | 1 day | 2.70 ± 0.00 | 2.14 ± 0.00 | 90.30 ± 0.09 | 0.65 ± 0.04 | 0.27 ± 0.02 | 1.06 ± 0.05 | |
| 2 days | 2.50 ± 0.15 | 1.73 ± 0.58 | 90.00 ± 0.89 | 0.60 ± 0.02 | 0.22 ± 0.00 | 0.89 ± 0.05 |
| Dry Weight of Precipitate after Ca(OH)2 Treatment | ||||||
|---|---|---|---|---|---|---|
| Dose of Ca(OH)2 (mg/mL) | 6.7 | 20 * | 30 | 46 | 60 | 80 |
| Dry weight (mg/mL) | 14 | 45 | 54 | 56 | 73 | 99 |
| Dry Weight of Precipitate after NaTPB Treatment | ||||||
| Dose of NaTPB (mg/mL) | 0.25 | 0.5 | 1 | 1.5 | 2 * | 2.5 |
| Dry weight (mg/mL) | 0.4 | 0.62 | 0.121 | 1.61 | 2.37 | 2.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-M.; Yoon, I.-H.; Kim, I.; Kim, J.-H.; Park, S.-J. Hydrothermal Desorption of Cs with Oxalic Acid from Hydrobiotite and Wastewater Treatment by Chemical Precipitation. Energies 2020, 13, 3284. https://doi.org/10.3390/en13123284
Kim S-M, Yoon I-H, Kim I, Kim J-H, Park S-J. Hydrothermal Desorption of Cs with Oxalic Acid from Hydrobiotite and Wastewater Treatment by Chemical Precipitation. Energies. 2020; 13(12):3284. https://doi.org/10.3390/en13123284
Chicago/Turabian StyleKim, Sung-Man, In-Ho Yoon, Ilgook Kim, June-Hyun Kim, and So-Jin Park. 2020. "Hydrothermal Desorption of Cs with Oxalic Acid from Hydrobiotite and Wastewater Treatment by Chemical Precipitation" Energies 13, no. 12: 3284. https://doi.org/10.3390/en13123284