Conceptualization of CO2 Terminal for Offshore CCS Using System Engineering Process
Abstract
:1. Introduction
2. Description of CCS Chain
3. System Engineering Process
3.1. Requirement Analysis
3.2. Functional Analysis
3.2.1. Functional Flow Block Diagram (FFBD)
3.2.2. Integration Definition for Function Modeling (IDEF0)
3.2.3. Timeline Analysis (TLA)
3.3. Design Synthesis
4. Results
4.1. CO2 Carrier and CO2 Terminal Requirements
4.1.1. Time for Carrier Round Trip and the Availability of Transport
4.1.2. Number of Carriers
4.1.3. Size of Carrier
4.1.4. Size and Number of Storage Tank in Terminal
4.1.5. The Estimation of the Amount of BOG in the CO2 Storage Tank at Terminal
4.2. Functional Analysis of the CO2 Terminal
4.2.1. FFBD of CO2 Transport Chain
4.2.2. Integration Definition for Function Modeling (IDEF0) of CO2 Terminal
4.2.3. Timeline Analysis (TLA) of the CO2 Storage Tank Operation
4.3. Identification of Physical Equipment of CO2 Terminal
5. Discussion
5.1. Process Flow Block Diagram of CO2 Terminal
5.2. Comparison with LNG Terminal
- (1)
- Differences in the number of storage tanks due to the size limit: LNG carriers usually travel long distances and convey a large amount of LNG, so this necessitates a large amount of LNG storage. However, relatively less CO2 storage is required in a CO2 terminal. Since the LNG tanks are operated in cryogenic and atmospheric pressure conditions except for small-scale satellite terminals, one LNG storage tank can be as large as ~160,000 m3 [56]. However, the pressure of the CO2 storage tank should be higher than the triple-point pressure of 5.1 bar, meaning the CO2 storage tanks are pressurized tanks, which are hard to manufacture at capacity bigger than around 5000 tons. This means the CO2 terminal requires multiple tanks, and in many cases, the carrier-transported CO2 has to be unloaded into several storage tanks. In this study, the carrier transports ~9000 tons of CO2, which is unloaded into two tanks that are 4500 tons each. Because overfill is one of the common causes of operational accidents [57], the multiple tanks in the CO2 terminal pose a high risk of overfill. Therefore, ensuring a high level of safety is necessary.
- (2)
- Importance of vaporizer: The storage time in an LNG terminal is usually longer than in a CCS terminal due to the longer carrier transport cycle. In this case, the amount of BOG generated in LNG carriers or storage tanks is huge and needs to be re-liquefied at a high cost and requiring considerable amounts of energy [58]. However, the CO2 carrier transportation cycle and temporary storage period in the terminal are relatively short. In our study, the carrier-transported CO2 is sent to the offshore pipeline within six days according to the TLA results. The amount of BOG in the CO2 terminal is less than the required vapor for preventing the decrease in pressure in the CO2 storage tanks as LCO2 is discharged to the pipeline. Therefore, the vaporizer in a CO2 terminal is essential.
5.3. Cost Comparison between One and Two Carriers
6. Conclusions
- (1)
- FFBD was used to identify the seven basic functions of the CO2 terminal: ‘Unload LCO2′, ‘Load VCO2′, ‘Receive LCO2′, ‘Store LCO2′, ‘Transmit LCO2′, ‘Generate VCO2′, and ‘Increase LCO2 T&P’. Then, IDEF0 was used to identify the correlation between these functions.
- (2)
- The short and repetitive CO2 carrier transport affects the configuration of the CO2 terminal. The CO2 terminal here is operated with a three-day cycle and at least four 4500-ton storage tanks are needed. The four tanks allow for continuous CO2 transmission to an offshore pipeline even if a CO2 carrier could not arrive at the CO2 terminal due to the bad weather for 2.5 days. This operational concept of a CO2 terminal was verified by the TLA.
- (3)
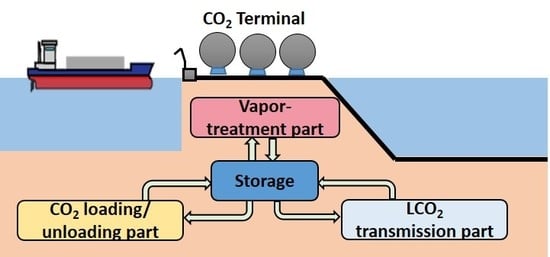
- A process flow block diagram was derived from the results of the functional analysis and design synthesis. The configuration of the CO2 terminal consists of a CO2 loading/unloading part, an LCO2 transmission part, and a VCO2 treatment part. These results were used in the subsequent design phase.
- (4)
- In this study, VCO2 is required for two purposes. The first is for filling the carrier cargo tank with VCO2 when unloading LCO2 from the CO2 carrier. In this case, the required VCO2 is covered by displaced VCO2 as LCO2 fills the storage tanks in the terminal. The second purpose is for controlling the pressure in the storage tank that is transmitting LCO2 to offshore pipelines. The required VCO2 is supplied from BOG from the other three storage tanks and vaporized CO2 from the small portion of discharged LCO2.
- (5)
- The comparison of our results with an LNG terminal indicated that a vaporizer is important in the CO2 terminal. As mentioned above, the BOG alone cannot meet the required amount for discharging the VCO2 in a storage tank. Therefore, it is necessary to pay more attention to vaporization rather than reliquefaction in the CO2 terminal. The CO2 terminal requires multiple small-sized storage tanks, unlike the LNG terminal.
- (6)
- Major design factors, such as the number and capacity of storage tanks at terminal, change depending on the number of carriers. Therefore, in order to minimize costs, the design parameters of the carrier and the terminal must be considered simultaneously.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IEA. Energy Technology Perspectives 2016; OECD: Paris, France, 2015. [Google Scholar]
- Budinis, S.; Krevor, S.; Dowell, N.M.; Brandon, N.; Hawkes, A. An assessment of CCS costs, barriers and potential. Energy Strategy Rev. 2018, 22, 61–81. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; p. 422. [Google Scholar]
- Jung, J.-Y.; Huh, C.; Kang, S.-G.; Seo, Y.; Chang, D. CO2 transport strategy and its cost estimation for the offshore CCS in Korea. Appl. Energy 2013, 111, 1054–1060. [Google Scholar] [CrossRef]
- Yoo, B.-Y. The development and comparison of CO2 BOG re-liquefaction processes for LNG fueled CO2 carriers. Energy 2017, 127, 186–197. [Google Scholar] [CrossRef]
- Kang, K.; Huh, C.; Kang, S.-G.; Baek, J.-H.; Noh, H.J. Estimation of CO2 Pipeline Transport Cost in South Korea Based on the Scenarios. Energy Procedia 2014, 63, 2475–2480. [Google Scholar] [CrossRef]
- Wetenhall, B.; Race, J.; Aghajani, H.; Fernandez, E.S.; Naylor, M.; Lucquiaud, M.; Chalmers, H. Considerations in the Development of Flexible CCS Networks. Energy Procedia 2017, 114, 6800–6812. [Google Scholar] [CrossRef]
- Spitz, T.; Avagyan, V.; Ascui, F.; Bruce, A.R.W.; Chalmers, H.; Lucquiaud, M. On the variability of CO2 feed flows into CCS transportation and storage networks. Int. J. Greenh. Gas Control 2018, 74, 296–311. [Google Scholar] [CrossRef]
- Kang, K.; Seo, Y.; Chang, D.; Kang, S.-G.; Huh, C. Estimation of CO2 Transport Costs in South Korea Using a Techno-Economic Model. Energies 2015, 8, 2176. [Google Scholar] [CrossRef]
- Seo, Y.; Chang, D.; Jung, J.-Y.; Huh, C.; Kang, S.-G. Economic Evaluation of Ship-based CCS with Availability. Energy Procedia 2013, 37, 2511–2518. [Google Scholar] [CrossRef]
- Yoo, B.-Y.; Choi, D.-K.; Kim, H.-J.; Moon, Y.-S.; Na, H.-S.; Lee, S.-G. Development of CO2 terminal and CO2 carrier for future commercialized CCS market. Int. J. Greenh. Gas Control 2013, 12, 323–332. [Google Scholar] [CrossRef]
- Yoo, B.-Y.; Lee, S.-G.; Rhee, K.-p.; Na, H.-S.; Park, J.-M. New CCS system integration with CO2 carrier and liquefaction process. Energy Procedia 2011, 4, 2308–2314. [Google Scholar] [CrossRef]
- Knoope, M.M.J.; Ramírez, A.; Faaij, A.P.C. Investing in CO2 transport infrastructure under uncertainty: A comparison between ships and pipelines. Int. J. Greenh. Gas Control 2015, 41, 174–193. [Google Scholar] [CrossRef]
- Brown, M.; Baddeley, A.; Coulthurst, A.; Taylor, S. The East Irish Sea CCS Cluster: A Conceptual Design; Eunomia Research & Consulting Ltd: Bristol, UK, 2011; pp. 1–19. [Google Scholar]
- Vermeulen, T.N. CO2 Liquid Logistics Shipping Concept (LLSC) Overall Supply Chain Optimization; Global CCS Institute: Canberra, Australia, 2011. [Google Scholar]
- Lauri, K.; Jouko, R.; Nicklas, N.; Sebastian, T. Scenarios and new technologies for a North-European CO2 transport infrastructure in 2050. Energy Procedia 2014, 63, 2738–2756. [Google Scholar] [CrossRef]
- Kujanpää, L.; Teir, S. Implications of the New EU Maritime Emission Monitoring Regulation on Ship Transportation of CO2. Energy Procedia 2017, 114, 7415–7421. [Google Scholar] [CrossRef]
- Brownsort, P. Ship Transport of CO2 for Enhanced Oil Recovery—Literature Survey; Murchison House: Edinburgh, UK, 2015. [Google Scholar]
- Emrah durusut, M.J. Shipping CO2—UK Cost Estimation Study; Suite 1 Bishop Bateman Court, Thompson’s Lane: Cambridege, UK, 2018. [Google Scholar]
- Furre, A.K.; Meneguolo, R.; Ringrose, P.; Kassold, S. Building confidence in CCS: From sleipner to the northern Lights project. First Break 2019, 37, 81–87. [Google Scholar]
- Lee, U.; Lim, Y.; Lee, S.; Jung, J.; Han, C. CO2 Storage Terminal for Ship Transportation. Ind. Eng. Chem. Res. 2012, 51, 389–397. [Google Scholar] [CrossRef]
- Riis, F. Norway CCS demonstration project: Evaluation of Jurassic reservoirs for safe CO2 injection and storage. In Proceedings of the 5th CO2 Geological Storage Workshop, Utrecht, The Netherlands, 21–23 November 2018. [Google Scholar]
- ZEP. The Costs of CO2 Transport; Zero Emission Platform: Brussels, Belgium, 2011. [Google Scholar]
- Northern Lights—Part of The Full-Scale CCS Project in Norway. Available online: https://northernlightsccs.com/en/about (accessed on 15 October 2019).
- Seo, Y.; Huh, C.; Lee, S.; Chang, D. Comparison of CO2 liquefaction pressures for ship-based carbon capture and storage (CCS) chain. Int. J. Greenh. Gas Control 2016, 52, 1–12. [Google Scholar] [CrossRef]
- Suzuki, T.; Toriumi, M.; Sakemi, T.; Masui, N.; Yano, S.; Fujita, H.; Furukawa, H. Conceptual Design of CO2 Transportation System for CCS. Energy Procedia 2013, 37, 2989–2996. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on Carbon Capture and Storage (CCS) processes: A review. Energy Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Kokubun, N.; Ko, K.; Ozaki, M. Cargo Conditions of CO2 in Shuttle Transport by Ship. Energy Procedia 2013, 37, 3160–3167. [Google Scholar] [CrossRef]
- Miyazaki, T.; Osawa, H.; Matsuura, M.; Ohta, M.; Ozaki, M. Offshore Operational Availability of Onboard Direct Injection of CO2 into Sub-seabed Geological Formations. Energy Procedia 2013, 37, 3168–3175. [Google Scholar] [CrossRef]
- Nakazawa, N.; Kikuchi, K.; Ishii, K.-I.; Yamaguchi, T.; Ohta, M.; Ozaki, M. Ship-based CO2 Injection into Subseabed Geological Formations Using a Flexible Riser Pipe Pickup System. Energy Procedia 2013, 37, 3176–3183. [Google Scholar] [CrossRef]
- Ozaki, M.; Ohsumi, T.; Kajiyama, R. Ship-based Offshore CCS Featuring CO2 Shuttle Ships Equipped with Injection Facilities. Energy Procedia 2013, 37, 3184–3190. [Google Scholar] [CrossRef]
- Aneziris, O.N.; Papazoglou, I.A.; Konstantinidou, M.; Nivolianitou, Z. Integrated risk assessment for LNG terminals. J. Loss Prev. Process Ind. 2014, 28, 23–35. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y. Dynamic optimization of the Boil-Off Gas (BOG) fluctuations at an LNG receiving terminal. J. Nat. Gas Sci. Eng. 2016, 30, 322–330. [Google Scholar] [CrossRef]
- Trotter, I.M.; Gomes, M.F.M.; Braga, M.J.; Brochmann, B.; Lie, O.N. Optimal LNG (liquefied natural gas) regasification scheduling for import terminals with storage. Energy 2016, 105, 80–88. [Google Scholar] [CrossRef]
- Bisen, V.S.; Karimi, I.A.; Farooq, S. Dynamic Simulation of a LNG Regasification Terminal and Management of Boil-off Gas. In Computer Aided Chemical Engineering; Eden, M.R., Ierapetritou, M.G., Towler, G.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44, pp. 685–690. [Google Scholar]
- Nagesh Rao, H.; Karimi, I.A. Optimal design of boil-off gas reliquefaction process in LNG regasification terminals. Comput. Chem. Eng. 2018, 117, 171–190. [Google Scholar] [CrossRef]
- Noh, Y.; Kim, J.; Kim, J.; Chang, D. Economic evaluation of BOG management systems with LNG cold energy recovery in LNG import terminals considering quantitative assessment of equipment failures. Appl. Therm. Eng. 2018, 143, 1034–1045. [Google Scholar] [CrossRef]
- Rao, H.N.; Karimi, I.A.; Farooq, S. Optimal Design of Boil-Off Gas Liquefaction in LNG Regasification Terminals. In Computer Aided Chemical Engineering; Eden, M.R., Ierapetritou, M.G., Towler, G.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44, pp. 2407–2412. [Google Scholar]
- Yoon, B.; Shin, J.; Lee, S. Technology assessment model for sustainable development of LNG terminals. J. Clean. Prod. 2018, 172, 927–937. [Google Scholar] [CrossRef]
- George, J.J.; Renjith, V.R.; George, P.; George, A.S. Application of fuzzy failure mode effect and criticality analysis on unloading facility of LNG terminal. J. Loss Prev. Process Ind. 2019, 61, 104–113. [Google Scholar] [CrossRef]
- Khan, M.S.; Effendy, S.; Karimi, I.A.; Wazwaz, A. Improving design and operation at LNG regasification terminals through a corrected storage tank model. Appl. Therm. Eng. 2019, 149, 344–353. [Google Scholar] [CrossRef]
- Pokkatt, P.P.; Srinivasan, R. Effect of Ambient Conditions on Boil Off Gas Generation in LNG regasification terminals. In Computer Aided Chemical Engineering; Kiss, A.A., Zondervan, E., Lakerveld, R., Özkan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 46, pp. 445–450. [Google Scholar]
- Reddy, H.V.; Bisen, V.S.; Rao, H.N.; Dutta, A.; Garud, S.S.; Karimi, I.A.; Farooq, S. Towards energy-efficient LNG terminals: Modeling and simulation of reciprocating compressors. Comput. Chem. Eng. 2019, 128, 312–321. [Google Scholar] [CrossRef]
- DoD. Systems Engineering Fundamentals: Supplementary Text; Systems Management College: Fort Belvoir, VA, USA, 2001. [Google Scholar]
- Grady, J.O. System Requirements Analysis, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 1–801. [Google Scholar]
- NASA. NASA Systems Engineering Handbook; NASA Headquarters: Washington, DC, USA, 2007.
- Grady, J.O. System Requirements Analysis; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Spitz, T.; Chalmers, H.; Ascui, F.; Lucquiaud, L. Operating Flexibility of CO2 Injection Wells in Future Low Carbon Energy System. Energy Procedia 2017, 114, 4797–4810. [Google Scholar] [CrossRef]
- IEAGHG. Operational Flexibility of CO2 Transport and Storage; IEAGHG: Cheltenham, UK, 2016. [Google Scholar]
- DNV. CO2RISKMAN. Guideance on CCS CO2 Safety and Environment, Major Accident Hazard Risk Management, Level 4—Specific CCS Chain Guidance; DNV-GL: Oslo, Norway, 2013; p. 307. [Google Scholar]
- Incropera, F.P. Fundamentals of Heat and Mass Transfer; John Wiley: Hoboken, NJ, USA, 2007; p. 1032. [Google Scholar]
- ASME. BPVC Section VIII-Rules for Construction of Pressure Vessels Division 2-Alternative Rules; The American Society of Mechanical Engineers: New York, NY, USA, 2015. [Google Scholar]
- Yoo, B.-Y. An Experimental Study on the Thermocline Layer in a Cargo Tank of CO2 Carriers; Seoul National University: Seoul, Korea, 2011. [Google Scholar]
- Thomas, K. What Are the Prospects for LNG-Carrier Tonne-Mile Demand to 2020? Available online: http://www.lngworldshipping.com/news/view,what-are-the-prospects-for-lngcarrier-tonnemile-demand-to-2020_46622.htm (accessed on 2 February 2018).
- Niu, W.C.; Li, G.L.; Ju, Y.L.; Fu, Y.Z. Design and analysis of the thermal insulation system for a new independent type B LNG carrier. Ocean Eng. 2017, 142, 51–61. [Google Scholar] [CrossRef]
- Lv, D.; Tan, W.; Liu, L.; Zhu, G.; Peng, L. Research on maximum explosion overpressure in LNG storage tank areas. J. Loss Prev. Process Ind. 2017, 49, 162–170. [Google Scholar] [CrossRef]
- Casal, A.; Olsen, H. Operational risks in QRAs. Chem. Eng. Trans. 2016, 48, 589–594. [Google Scholar]
- Tan, H.; Zhao, Q.; Sun, N.; Li, Y. Enhancement of energy performance in a boil-off gas re-liquefaction system of LNG carriers using ejectors. Energy Convers. Manag. 2016, 126, 875–888. [Google Scholar] [CrossRef]









| No. | Functions | Requirements | Equipment | |
|---|---|---|---|---|
| 5.0 and 11.0 | Transport LCO2 by carrier and Return carrier to capture site | (1) Number of carriers = 2 (2) One-way transport time ≤ 1 day (3) Availability of carriers < 80% (4) Amount of CO2 per one carrier = 8549 tons | Two 9000-ton CO2 carriers | |
| 6.1 | Unload LCO2 | (1) Amount of unloaded LCO2 = 8549 tons (2) Time required for unloading ≤ 4 h | LCO2 unloading system | |
| 6.2 | Load VCO2 | (1) Amount of loaded VCO2 = 329 tons (2) Time required for loading ≤ 4 h | VCO2 extracting system VCO2 loading system | |
| 7.1 | Receive LCO2 | (1) The size of 1 storage tank = 4500 tons | 4500-ton storage tanks | |
| 7.2 | Store LCO2 | (1) Temperature and pressure CO2 in storage tank are maintained at −27 ℃ and 16 bar, respectively, by removing BOG (2) Buffer capacity = 60 h’ injection amount (3) The required number of CO2 tanks = 4 | BOG removal system Four 4500-ton storage tanks | |
| 7.3 | Transmit LCO2 | (1) Continuous extraction rate of LCO2 from storage tank ≥ 2740 tons/day | LP Pump | |
| 7.4 | Generate VCO2 | (1) Daily required amount of VCO2 = 109.7 tons (2) Prevent decrease in pressure of storage tanks by charging VCO2 | VCO2 generating system VCO2 charging system | |
| 8.1 | Increase LCO2 T & P | (1) Target temperature = 3–5 ℃ (2) Target pressure ≥ 120 bar | Heat exchanger Booster pump | |
| No. of Carriers | One Carrier | Two Carriers | |
|---|---|---|---|
| Carrier | Size of one carrier (tons) | 15,000 | 9000 |
| CAPEX ($) | 51.7 M | 76.2 M | |
| Capital Recovery Cost ($) | 2.8 M | 4.0 M | |
| OPEX ($) | 4.4 M | 6.0 M | |
| Annual cost of carrier ($) | 7.2 M | 10.0 M | |
| Terminal | No. of storage tanks | 7 | 4 |
| Size of storage tank (tons) | 3800 | 4500 | |
| CAPEX of Storage tanks ($) | 14.9 M | 9.5 M | |
| Capital Recovery Cost ($) | 1.0 M | 0.6 M | |
| OPEX of storage tanks ($) | 0.7 M | 0.5 M | |
| Annual cost of storage tanks ($) | 1.7 M | 1.1 M | |
| Total | Sum of annual costs of carriers and storage tanks at terminal ($) | 8.8 M | 11.0 M |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, H.; Kang, K.; Huh, C.; Kang, S.-G.; Han, S.J.; Kim, H. Conceptualization of CO2 Terminal for Offshore CCS Using System Engineering Process. Energies 2019, 12, 4350. https://doi.org/10.3390/en12224350
Noh H, Kang K, Huh C, Kang S-G, Han SJ, Kim H. Conceptualization of CO2 Terminal for Offshore CCS Using System Engineering Process. Energies. 2019; 12(22):4350. https://doi.org/10.3390/en12224350
Chicago/Turabian StyleNoh, Hyonjeong, Kwangu Kang, Cheol Huh, Seong-Gil Kang, Seong Jong Han, and Hyungwoo Kim. 2019. "Conceptualization of CO2 Terminal for Offshore CCS Using System Engineering Process" Energies 12, no. 22: 4350. https://doi.org/10.3390/en12224350