1. Introduction
Growth of the demand in seawater and brackish water desalination over the past few years has been motivated by increasing water stress in an increasing number of countries. Even if a major part of desalination systems is powered by fossil energies, desalination systems driven by renewable energies are now well-known and available on the market. Solar desalination is particularly interesting, considering how the most water-lacking areas are coastal and have an important sun resource.
Solar-driven desalination can be led in two different ways: distillation or membrane separation. In solar distillation systems, the water is evaporated by the solar heat. The most current distillation-based technologies are the multi-effect distillation (MED) and the multi-stage flash distillation (MSF). Those processes have a total specific primary energy consumption (SEC) commonly ranging between 30 and 80 kWh per cubic meter of produced clean water [
1]. The consumed energy is, in this case, mostly thermal, but some processes include mechanical circulation or compression devices. In this case, the total primary energy can be assessed by taking into account an energy equivalent of mechanical energy to thermal energy, of which ratio is often taken to be approximately 3 kWh
th/kWh
el.
Membranes-based desalination consists in separating the salt from the water through a semi-permeable membrane. The most-implemented membrane separation processes are electrodialysis and reverse osmosis (RO). In electrodialysis processes, the salt is drained through an ion-selective membrane by way of two electrodes submitted to electrical potential. This process needs electrical energy to operate, and leads to an energy consumption lower than 2 kWh
elec·m
−3. The process is more appropriate for brackish water desalination with a salinity below 2 g·L
−1 [
2,
3].
Reverse osmosis is a membrane separation technique which is widely used for desalination and food applications. RO desalination represents 65% of worldwide desalination systems [
4], mainly for its high performance and relatively low production cost. In RO processes, the pressurized feed water flows through a semi-permeable membrane which retains the salt ions and only lets water go through. In order to make this permeation possible, the feed water has to be pressurized beyond its osmotic pressure, which depends on the salt concentration and water temperature. The RO technology leads to specific energy consumptions ranging from 2 to 10 kWh
mec·m
−3 [
5], and depends on the water salinity (from 2 to 40 g·L
−1) and temperature. This low energy consumption is obtained by recovering the hydraulic energy of the highly pressurized concentrated water at the membrane output by means of energy recovery devices, such as turbines or pressure exchangers. Distillation-based systems are easier to set up, but their energy consumptions are higher than membrane-based technologies. Membranes-based desalination enables low energy consumption in comparison to distillation processes, but they have some major operating issues, such as membrane deterioration or fouling, that can lead to higher operating costs. Nevertheless, reverse osmosis is an interesting technology because of its low energy needs and its potential to be implemented at a wider scale [
6]. Solar photovoltaic-driven RO processes (PV-RO) have been widely developed nowadays, thanks to the modularity of these two components and the price drop of photovoltaics panels [
7]. Recently, more interest has been brought to battery-less PV-RO that is more eco-friendly and has the benefit of lower investment costs [
8,
9]. Nevertheless, exploiting solar energy via photovoltaic panels in order to produce electricity, later converted into hydraulic energy, implies the use of high-pressure pumps and associated control devices that leads to important losses of efficiency [
10]. These losses are mostly due to successive energy conversion. Furthermore, intermittent operation has been proven to increase biofouling because of the water non-circulation times [
11]. Solar heat-driven RO-based systems obtained by coupling a thermal solar collector to a di-thermal power cycle and a RO unit could be an interesting solution to reduce energy conversion losses, and thus to obtain better energy efficiencies [
12,
13]. Manolakos et al. studied and experimented with a solar-driven RO desalination unit by implementing an Organic Rankine Cycle (ORC) [
14]. They obtained relatively interesting performances despite the losses from its long energy conversion chain composed from expanders and pumps. They showed that solar thermal-driven reverse osmosis could be a competitive desalination technique. To avoid this kind of loss, another ORC-RO process was developed by Igobo et al. [
15]. In their process, the expansion energy is directly transmitted to the feed water by a cylinder, and it also has very low mechanical energy consumption. Another similar process was developed by Nihill et al. in 2018, where they coupled a thermal water pump to a RO membrane [
16]. In their study, the working fluid expansion is also realized in a cylinder with a mobile piston. In this process, the cylinder, which is directly heated by a heat exchanger, acts as an evaporator. This process allows desalination of the brackish water with a salinity of about 1.1 g·L
−1 by reaching 2.2 bars of pressure. However, this work is still under development, and shows high thermal energy consumption since it presents high thermodynamic irreversibilities due to pressurization and depressurization phases in the cylinder, and also does not include any mechanical energy recovery devices yet on the brine.
With the same guiding idea, an innovative thermo-hydraulic process is described and investigated in this paper. This process also enables the direct use of the expansion work of a fluid that follows a thermodynamic engine cycle, in order to pressurize the saline water in the RO unit. Importantly, this solution enables the reduction of energy conversion losses during the mechanical work conversion, and improves the efficiency of the global process. The process implements simple hydraulic and thermal components in order to be cost-effective, as well as easy to maintain and to operate. It can be powered by low-grade heat, such as solar heat provided by common flat-plate solar collectors. With these features, the process is able to operate in off-grid, remote areas. However, such a process generates a highly dynamic evolution of the pressure that is imposed to the RO membrane due to the sun irradiation variability and the cyclic behavior of the thermo-hydraulic engine. This pressure evolution consists in cyclic changes of pressure lasting a few minutes, where each cycle is characterized by a step change in pressure, followed by a plateau, and then a progressive decrease. The impact of these unsteady operating conditions on the RO unit performance has to be evaluated, and requires precise modelling that enables assessment of the effects of this pressure variation on the RO membrane performance.
The impact of dynamic applied pressure on the RO membrane, which affects its performances, is not yet well-known. Only a few studies on RO process operations in transient running conditions have been led, and these concluded only that the membrane performance was significantly affected by these dynamic operating conditions. Several linked phenomena have been reported, such as membrane compaction [
17], higher salt accumulation at the membrane wall, and development of a greater diffusive layer in the feed water channel [
18]. The latter phenomenon, called polarization, is well-known when a constant pressure is applied [
19]. Yet, studies treating it in dynamic running conditions are not enough to predict the influence of dynamic pressure constraints, which are characteristic of the process studied in this paper.
Some studies have been led on RO processes operating under variable pressure constraints for several applications, such as photovoltaic-powered RO [
17] or wastewater treatment [
20]. They showed that dynamic running conditions have an impact on the salt concentration profile at the membrane wall which affects the permeation rate. Different kinds of transient modeling have been developed mainly with the assumption of a stationary diffusive layer that is established according to the well-known film theory. Rodgers et al. [
21] developed dynamic modeling, and led experiments on an ultrafiltration process with a membrane submitted to pressure pulses. They studied the influence of these pressure pulses on polarization effects, and showed that short pulses of negative transmembrane pressure may increase the permeate flux and minimize the polarization effects. Kim et al. [
22] compared the film theory results obtained by an analytical model and a two-dimensional numerical convection–diffusion model. This study, which has been experimentally validated, showed the relevance of the film theory model for steady working conditions. A dynamic model for tubular membranes was also introduced by Ali et al. [
23] using a mathematical approach, and showed that a high step-change of the feed mass flow rate does impact the RO membrane behavior significantly. Besides these modeling studies, more practical researches have been made on RO desalination driven by unstable renewable energy sources. Cheddie et al. [
24] studied a RO process powered by wave energy, and introduced dynamic modeling of the RO unit. They also showed that a periodic applied pressure may minimize the polarization effects and enhance the performances. Such works suggests that dynamic running conditions impact on RO membrane performances, and the membrane behavior needs to be modeled in a dynamic way in order to assess these impacts.
In this paper, a new solar heat-driven RO desalination process is described, and a dynamic modelling of the whole process is developed. In order to take the impacts of the cyclic pressure changes applied to the membrane into account, a 2D dynamic modelling of RO membrane is implemented. This membrane modelling enables a better understanding of the dynamic establishment of the polarization layer. An experimental study has also been carried out on a test bench designed for brackish water desalination under variable pressure evolutions and controlled mass flow rates of the brine in order to allow for a comparison with model results. Experimental data obtained with steady pressure tests are used to identify the parameter of the dynamic model. The experimental results obtained under dynamic operating tests are compared to numerical results in order to validate the dynamic modelling. Simulations of the whole process have been performed to study its dynamic behavior and analyze its performances.
2. Description of the Thermo-Hydraulic Desalination Process
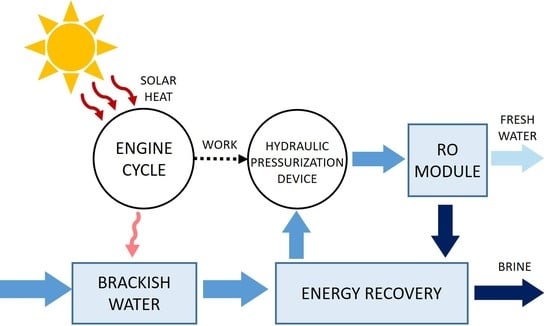
The thermo-hydraulic desalination process is described
Figure 1. It results from a direct coupling of a solar-powered, Rankine-like engine cycle with a RO module.
The engine cycle is composed of an evaporator heated by solar flat-plate collectors, and a condenser cooled by the saltwater to be desalinated. The high-pressure vapor of the working fluid that is generated by the evaporator flows into a first transfer tank filled with feed water and pressurizes it above its osmotic pressure. The condenser enables the condensation of the working fluid previously accumulated in a second low-pressure tank.
The pressurization of the water in the tank is realized thanks to an elastically deformable membrane which transfers the mechanical energy from the high-pressure working fluid to the feed water. The working fluid vapor is produced by the evaporator at a pressure ranging from 10 to 20 bars, thanks to the heat delivered at about 50 to 80 °C by the solar collectors. The suitable working fluid for these required operating conditions and for the inflammability criterion has been found to be the sulfur dioxide (SO2).
The hydraulic energy of the pressurized concentrated water (brine) flowing out of the RO module is recovered by a specific hydraulic cylinder (main actuator in
Figure 1), which enables it to pump and fill the low-pressure reservoir with saltwater. A second hydraulic actuator (refiling actuator in
Figure 1) is used to transfer the working fluid from the low-pressure condenser to the high-pressure evaporator. The main actuator is composed of three chambers in order to make it behave in a symmetric way in both directions of movement, that is to say, in order to ensure that the pumped and the pushed-back volumes of water inside the chambers are equal. The pressurization is done in the middle chamber that contains the driving piston, which transmits the mechanical energy and sets the piston in movement in the two lateral chambers in order to realize the pumping of the saltwater and the filling of the transfer tank. Thanks to this main hydraulic cylinder, the feed water to be desalinated is firstly pumped, then goes through the condenser, thus acting as a cold source for the thermodynamic cycle, and is temporarily accumulated in one of the lateral chambers of the hydraulic cylinder. Simultaneously, as the piston moves, the water to be desalinated, which was previously accumulated in the second lateral chamber of the hydraulic cylinder, is pushed out and pressurized in order to fill the low-pressure water tank connected to the condenser. At the same time, the feed water that is pressurized in the other tank by the high-pressure vapor of the engine cycle flows through the RO membrane. Clean water (permeate) is produced and the brine, which is still at high pressure, is used to drive the above-mentioned hydraulic cylinder.
Several distributors and valves allow for the role inversion of the reservoirs and the brine distribution in the process, as well as the control and cycling of the different operating steps of the process. The brine flow is controlled at the main cylinder outlet by a constant flow rate valve, which thus regulates the moving velocity of the cylinder piston.
The other independent actuator, which ensures the pressurization and the transfer of the liquid working fluid from the low-pressure condenser to the high-pressure evaporator tank, is set in motion by high-pressure vapor from the evaporator. The depressurized vapor in this cylinder flows into the condenser.
2.1. Description of the Cycle Phases
The operating cycle of the process consists of several steps described in
Figure 2. An initialization phase enables pressurization at the high-pressure P
h of the transfer tank 1, which is already completely filled with saltwater, by connecting it to evaporator. Note that the high operating pressure has to be beyond the feed-water osmotic pressure to overcome transfer resistance throughout the RO membrane.
During the first step, or alpha phase (
Figure 2a), the transfer tank 1 is linked to the evaporator, and the transfer tank 2 is connected with the condenser. The high-pressure feed water contained in the transfer tank 1 goes through the RO module and produces clean water under ideally constant pressure, P
h. The still-pressurized output brine is recovered and used by the main actuator to pump the saline water and fill the transfer tank 2 that is at the condenser pressure, P
l. The water filling the transfer tank 2 pushes out the vapors of the working fluid from that tank to the condenser, where it condenses at low pressure.
In the second step, or beta phase (
Figure 2b), the transfer tank 1 is disconnected from the evaporator. Thus, the tank is no longer fed with high-pressure vapor. Nevertheless, the pressurized vapor contained in this tank continues to push out the saltwater to the RO membrane, generating an expansion of the gas (increasing the gas volume) and thus a decrease of the pressure until a minimum allowable pressure, P
β. That phase is also a desalination phase, but under a decreasing pressure, and thus enables a decreasing clean water production. At the end of this step, the transfer tank 2 is completely filled with feed water that will be later desalinated, and all of the working fluid previously contained in it has been expelled into the condenser. At this point, a half-cycle has been achieved (
Figure 2c)—the first tank is then fully filled with vapor at the final pressure P
β of the beta phase, and the second one is full of saltwater at condenser pressure P
l. Distributors are then able to switch the tanks’ roles and run a new half-cycle.
Figure 2 also represents the volume and pressure evolutions of the working fluid vapor contained in each transfer tank. They respectively correspond to the feed tank volume V
α at the end of alpha phase at evaporator pressure P
h, the fully filled tank volume at the end of beta phase 2 at the end of phase pressure P
β, and the tank volume at condenser pressure P
l at the beginning of the next half-cycle after reversing the role of the feed tanks.
The design of the hydraulic cylinders (rod length, piston diameters, …) has been led in order to optimize the hydraulic energy recovery efficiency.
2.2. Refilling
Independently to the two presented phases, the refilling actuator permits refilling of the evaporator with liquid working fluid from the condenser. This cylinder is made with two chambers in which two pistons move with different diameters. The difference in the areas of the pistons allows for the pressure drop between the condenser and the evaporator to be overcome. The motor chamber is fed by the high-pressure vapor supplied by the evaporator, and sets the piston in motion (
Figure 3). Then, the piston in the receiving chamber pressurizes, pushes out the liquid to the evaporator, and simultaneously sucks up the liquid from the condenser, and fills the chamber with liquid working fluid to be pressurized later. This refilling phase is activated when the liquid level of working fluid in the liquid/vapor separation tank of the flooded-type evaporator is low, and takes place during the beta phase when transfer tanks are disconnected with the evaporator.
In order to analyze the behavior and the performances of this process, dynamic modeling of the whole process has been developed and described in the following section.
5. Process Simulations and Results
Once the dynamic modeling was fully established, it was implemented to assess the whole process behavior during a complete day. Simulation parameters were determined from a previous sizing, and simulations were made under static working conditions.
Before the RO model integration in the complete process modeling, an additional study was conducted on local variations along the membrane channels. This local study aimed to evaluate the impact on the membrane’s local performances of a pressure steep variation, similar to the one involved in the process.
5.1. Axial Concentration Evolution Study
The dynamic model also enables characterization of the longitudinal evolution of the concentrations’ profiles at the wall on each side of the membrane when the applied pressure varies, and thus the resulting evolution of the local permeate flux along the membrane.
The inlet feed pressure evolution was used to study the local effect in the feed channel along the membrane. The operating conditions are a feed flow rate of about 0.3 L·min
−1 and a feed concentration of about 4 g·L
−1. As described in
Figure 13 and specified in
Table 3, several time positions were chosen to assess the local impacts of the quickly decreasing pressure.
For a better comparison study, a membrane saturation ratio, Sm (Equation (46)) was introduced. It is defined as the ratio between local bulk membrane concentration and the maximum membrane concentration, C
max that would be obtained when the applied trans-membrane pressure equals the osmotic pressure, neglecting the permeate concentration (Equation (47)).
This saturation ratio can be understood as a saturation degree of the membrane in the channel. When Sm = 1, the maximal concentration is reached regarding the applied pressure; then there is no longer water transfer occurring across the membrane.
Figure 14 shows the local permeate flux and membrane saturation ratio evolutions for six time positions considered during the pressure-decreasing phase (corresponding to the beta phase of the desalination process). This case study enables assessment of the effects of a decreasing feed pressure on local membrane concentration and recovery rates.
From this preliminary study, it can be seen that the influence of a variable applied pressure on membrane wall concentration and the resulting permeate flow is not negligible. A decreasing evolution of the permeate production is observed along the membrane as the membrane concentration rises. Variations of the membrane saturation ratio are more important at the membrane input.
Several cycles are then simulated with a constant solar irradiation applied to the solar collector. Next, the process was simulated over a full day, by considering two data sets of real weather conditions: one sunny and one cloudy.
5.2. Operating and Geometrical Simulation Parameters of the Process
Firstly, size parameters were established to give a scale to the process. These parameters were determined with the aim of an average daily production of desalted water of 500 L·m
−² of solar collectors from brackish water with a salt concentration of 4 g·L
−1. Thermodynamic coefficients were chosen to fit the technical data from selected components. These parameters are summarized in
Table 4.
Operating conditions were also determined and gathered in
Table 5. Sulfur dioxide was selected as working fluid for its suitable saturation conditions, i.e., it gives high evaporator pressure with a low-grade heat temperature ranging from 50 °C to 80 °C. Feed salt concentration was chosen as typical brackish groundwater and lakes. The final pressure at the beta phase end was chosen to be around a value of 8 bars that maximize clear water production during a cycle.
Controlled brine flow at the outlet of the feed pressure recovery cylinder was determined from the pressure at the end of beta phase and the inlet evaporator power in order to ensure that the main actuator piston had completed its full stroke at the end of each half-cycle.
5.3. Simulations with a Constant Thermal Power Supplied to the Evaporator
In the first stage, simulations were realized over several cycles with a constant thermal power of 500 W supplied to the evaporator.
Figure 15 shows the pressure evolutions in the main components of the process. From this representation, the two main half-cycle phases clearly appear: the alpha phase, where the feed tank is maintained at high pressure, and the beta phase, where its pressure decreases since it is no longer linked to the evaporator. At the beginning of the alpha phase, the second tank is linked to the condenser, so its pressure suddenly drops to reach the condensation pressure. These results show the highly dynamic behavior of the process with half-cycles of about 50 s, shared between the alpha and beta phases. The refilling phase happens each three half-cycles, and can be identified from the pressure drop that occurs in the evaporator during its pressurization, that is, during the beta phase (here at 125 s).
It appears that the evaporator efficiently enables the pressurization of the reservoir to equal to nearly 15 bars, which is a pressure level sufficiently high enough to ensure the RO desalination of the brackish water with a salt concentration ranging from 3 to 8 g·L−1. It also appears that, during the first step, the pressurization is realized at a pressure that is almost constant, which is as expected, taking into account the dimensioning of the evaporator. The condenser pressure evolution shows that the mass flow rate of the pumped feed water is enough to cool it efficiently and maintain a stable condensing pressure. It appears that the temperature and pressure evolutions are similar, and varies between (298 K, 3.5 bars) in the condenser and (340 K, 15 bars) in the evaporator. The temperature of the evaporator and the condenser remains stable over the cycle, whereas the tanks’ ones decrease during the beta phase due to the vapor expansion. When the condenser is connected to the tank, the condenser temperature rises a little and stabilizes quickly.
In the permeate production plot presented in
Figure 16, the two working phases can also be identified. During the alpha phase, the permeate flow is quite stable even when a slight decrease can be noticed, meaning that the pressure applied to the membrane is unstable, as mentioned before. The decrease during the beta phase is, however, well-pronounced. Nevertheless, this flow decrease never drops to zero because the chosen end-pressure of the beta phase, here being 8 bars, is far beyond the feed-water osmotic pressure (3 bars).
Figure 17 shows the operation of the two actuators and their rods’ positions during the running cycles. The design of these actuators, that is, the piston surface design, has led to consider a total stroke of 0.2 m for the main actuator and 0.1 m for the refilling actuator. The main actuator rod is always in movement, oscillating between its initial position and its end stroke with a constant speed, as the mass flow rate of the brine is controlled and maintained to a constant value. This oscillating movement demonstrates the correct actuator sizing. The other actuator, the refilling one, is activated at every refilling phase, which takes place during a beta phase. It goes until its total stroke is reached, and remains in its position until the next refilling phase, activated by a low liquid level of working fluid in the evaporator.
This first study with a constant power source enables a better understanding of the dynamic of the cycles and offers a comparison between the simulations with the theoretical plots presented in
Figure 2. However, there exists a second type of dynamic perturbation, the one linked to the variation of the solar irradiation over the day, that may impact this behavior and the performances of the process. This then needs to be simulated in order to assess the process response over a full day.
5.4. Full-Day Simulation
A full-day simulation was thus carried out, and the resulting production and quality of fresh water have been represented aside from the solar irradiation evolution in
Figure 18. The data gives the global solar irradiation collected by a solar captor facing south, with an inclination of 40° at the latitude of Perpignan (France). It varies between 550 and 1000 W.m
−2, which corresponds to a power of 800 to 1500 W that is received by the 1.5 m
2 solar collector and a collected thermal power of 400 to 800 W that is transmitted to the evaporator. This first plot allows for an estimation of the simulated solar collector efficiency of about 50%. The second graph shows the evolution of the permeate flow averaged for each half-cycle during the day, thus demonstrating a trend which clearly correlates with the input thermal power of the system. The obtained recovery ratio of saltwater varies between 30 and 60% during the day. The last plot shows the fresh-water quality over the daily run. The produced water quality is also impacted by the solar power, but its variation is less noteworthy than the permeate production.
Figure 19 and
Figure 20, respectively, show the computed pressure and temperature evolutions of the evaporator over the day. The high pressure varies from 11 to 21 bars, which corresponds to a temperature range from 330 K to 360 K. It can be noticed that the evaporator pressure drop during the alpha phase rises with higher thermal power input. This is due to the increase of the vapor flow across the valve between the evaporator and the transfer tanks.
Such preliminary results allowed us to estimate the daily production of fresh water to 743 L, which corresponds to the community needs of about 5 to 10 families [
29]. A salt concentration of around 2.6 mg·L
−1 was obtained by this thermo-hydraulic process operating during 136 cycles with 1.5 m
2 of solar collectors under the climatic conditions of a very sunny day. The specific thermal energy consumption was evaluated to be about 5.8 kWh·m
−3 of produced fresh water, with daily fresh-water productivity of around 500 L per m
2 of thermal solar collectors. These performances seem to be competitive in comparison to other solar RO desalination processes that lead to similar energy performances [
30,
31].
To supplement this performance analysis of the process operating under stable climatic conditions, another simulation has been led considering a highly cloudy day. The results are plotted in
Figure 21. This test aims to determine the system reaction to thermal power perturbations at the evaporator. The studied day is disturbed by several cloudy periods, and the irradiation variates between 20 and 900 W·m
−2 with the same daytime duration as the sunny day simulation. The process starts when the evaporator pressure is sufficiently high (approximately 9.5 bars) and operates during almost all of the time period. In
Figure 21, the first plot shows solar power that is transmitted to the solar collector and the inlet evaporator power. During the cloudy periods, the evaporator and collector temperatures reach a thermal equilibrium, and then the evaporator inlet power drops to zero. The second plot shows the permeate production averaged over each half-cycle. The permeate flow follows the evaporator power over the day and is rarely null, thanks to the process inertia. The last plot shows the permeate salt concentration that oscillates between 2.5 and 3 mg·L
−1, and is higher for low inlet power. Missing points in this plot correspond to the process shutdown period, due to an evaporator pressure that is too low (lower than 9 bars).
Figure 22 and
Figure 23 respectively show the pressure and temperature evolutions in the evaporator simulated over the cloudy day. It can be noticed that the start of the process matches an evaporator temperature of about 325 K and a pressure of about 8.8 bars, and reaches a maximum of 350 K for 16 bars. These plots show that the evaporator is able to supply a pressure that is high enough for operating the RO module and the main cylinder despite the power disturbances, and enables the process to work for almost the entire day.
The results from this second simulation gives a daily production of 275 L with an average quality of about 2.7 mg·L−1 and a specific energy consumption of about 5.67 kWhth·m−3. The water production is much lower on cloudy days due to a lower solar energy input, but the simulation shows that the process inertia allows the production to almost continue over the day despite the disturbances of the source. The operation of the process facing irregular thermal power supply at the evaporator shows that performances could be improved by a continuous control of brine flow and end pressure of the beta phase. This kind of regulation can enable the process to run in the case of low evaporator pressure, and maximize water production during high evaporation pressure periods.
6. Conclusions
Directly powering an RO device by low-temperature heat through an integrated thermodynamic engine cycle without energy storage can lead to drastic reduction of installation, maintenance, and operating costs in comparison to current implemented PV-operated RO desalination plants. The solar-driven thermo-hydraulic process described in this study was designed for these objectives, and can be an interesting alternative for remote populations facing water and energy scarcity, thanks to its autonomous operation and the low cost of the implemented hydraulic and thermal components. This innovative process was presented in this paper, and its working has been described in detail.
Dynamic modeling of each component of this process has been established considering mass and energy balances under detailed hypothesis. Particular attention has been paid to reverse osmosis membrane modeling, since the dynamic behavior of RO membranes has not been studied much in the literature. An experimental and numerical study on the RO module operating in variable pressure conditions was then presented. An RO membrane adapted for brackish water desalination was experimentally tested under different pressure constraints to calibrate the dynamic model. The evolution of the recovery rate and brine dimensionless concentration obtained by the numerical model matched closely with the experimental one. An analysis of the recovery rate and the saturation ratio along the membrane also showed the high influence of the variability of the feed pressure on the diffusive layer establishment delay, thus impacting the permeate flow across the membrane. This demonstrates that precise dynamic modeling of the RO module is required to take into account the impacts of highly transient running conditions on this innovative, solar, thermally driven RO process.
The simulation of the whole process that has been carried out in this study showed that it is possible to produce approximately 750 L per day from 4 g·L
−1 of brackish water by such an installation by only implementing 1.5 m
2 of solar collectors operating at a low temperature (55 to 85 °C). These preliminary analyses demonstrate that this process may be competitive in comparison to other renewable energy driven desalination systems, especially to PV-RO autonomous processes with SEC of around 2–4 kWh
el·m
−3 [
32,
33]. A second simulation was also led in order to assess the process behavior during a cloudy day. The results show that the evaporator inertia minimizes the irradiation perturbation effects and allows for an almost continuous running over the day, but results in lower fresh-water productivity.
The numerical results are promising, but further simulations are necessary to optimize the process working parameters in the aim of lowering the thermal energy consumption, and to improve the process operations facing solar energy variations. A parametrical study also needs to be carried out in order to assess its technical and economical relevancy. An experimental set-up is currently in progress to validate this dynamic modeling and experimentally demonstrate the feasibility of the technical process.