Breakthrough and Episodic Cancer Pain from a Palliative Care Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Patients
2.3. Assessments
2.4. Statistical Analysis
2.5. Ethics
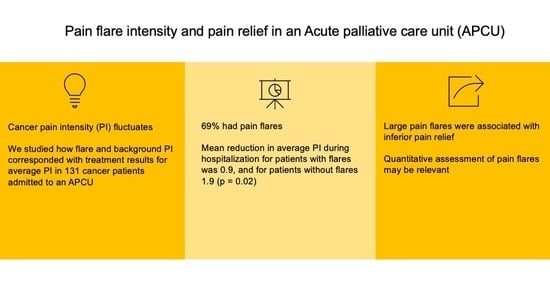
3. Results
3.1. Demographics
3.2. Pain Alleviation during Hospitalization for Patients with and without Pain Flares
3.3. Absolute Pain Spike Intensity and Pain Relief
3.4. Relative Pain Spike Intensity and Pain Relief
3.5. The Significance of Background Pain Intensity for Pain Control
3.6. Transmucosal Fentanyl Formulations and Parenteral Opioids in Patients with Pain Flares
4. Discussion
4.1. Statement of Principal Findings
4.2. Appraisal of Methods
4.3. Comparison with Previous Work
4.4. Clinical and Scientific Implications
4.5. Further Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van den Beuken-van Everdingen, M.H.J.; van Kuijk, S.M.J.; Janssen, D.J.A.; Joosten, E.A.J. Treatment of Pain in Cancer: Towards Personalised Medicine. Cancers 2018, 10, 502. [Google Scholar] [CrossRef]
- Thronaes, M.; Raj, S.X.; Brunelli, C.; Almberg, S.S.; Vagnildhaug, O.M.; Bruheim, S.; Helgheim, B.; Kaasa, S.; Knudsen, A.K. Is it possible to detect an improvement in cancer pain management? A comparison of two Norwegian cross-sectional studies conducted 5 years apart. Support. Care Cancer 2016, 24, 2565–2574. [Google Scholar] [CrossRef]
- Lohre, E.T.; Thronaes, M.; Brunelli, C.; Kaasa, S.; Klepstad, P. An in-hospital clinical care pathway with integrated decision support for cancer pain management reduced pain intensity and needs for hospital stay. Support. Care Cancer 2020, 28, 671–682. [Google Scholar] [CrossRef]
- Thronaes, M.; Lohre, E.T.; Kvikstad, A.; Brenne, E.; Norvaag, R.; Aalberg, K.O.; Moen, M.K.; Jakobsen, G.; Klepstad, P.; Solberg, A.; et al. Interventions and symptom relief in hospital palliative cancer care: Results from a prospective longitudinal study. Support. Care Cancer 2021, 29, 6595–6603. [Google Scholar] [CrossRef]
- Cleeland, C.S. The measurement of pain from metastatic bone disease: Capturing the patient’s experience. Clin. Cancer Res. 2006, 12, 6236s–6242s. [Google Scholar] [CrossRef]
- Haugen, D.F.; Hjermstad, M.J.; Hagen, N.; Caraceni, A.; Kaasa, S. Assessment and classification of cancer breakthrough pain: A systematic literature review. Pain 2010, 149, 476–482. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singapore 1994, 23, 129–138. [Google Scholar]
- Klepstad, P.; Loge, J.H.; Borchgrevink, P.C.; Mendoza, T.R.; Cleeland, C.S.; Kaasa, S. The Norwegian brief pain inventory questionnaire: Translation and validation in cancer pain patients. J. Pain Symptom Manag. 2002, 24, 517–525. [Google Scholar] [CrossRef]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]
- Mercadante, S.; Portenoy, R.K. Breakthrough cancer pain: Twenty-five years of study. Pain 2016, 157, 2657–2663. [Google Scholar] [CrossRef]
- Ballantyne, J.C. Breakthrough pain: Just pain? Pain 2016, 157, 2621–2622. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Park, M.; Shamieh, O.; Paiva, C.E.; Perez-Cruz, P.E.; Muckaden, M.A.; Bruera, E. Personalized symptom goals and response in patients with advanced cancer. Cancer 2016, 122, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I.; Committee, E.G. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Lohre, E.T.; Thronaes, M.; Klepstad, P. Breakthrough cancer pain in 2020. Curr. Opin. Support. Palliat. Care 2020, 14, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.N.; Dickman, A.; Reid, C.; Stevens, A.M.; Zeppetella, G. The management of cancer-related breakthrough pain: Recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur. J. Pain 2009, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Hagen, N.A. Breakthrough pain: Definition, prevalence and characteristics. Pain 1990, 41, 273–281. [Google Scholar] [CrossRef]
- Caraceni, A.; Bertetto, O.; Labianca, R.; Maltoni, M.; Mercadante, S.; Varrassi, G.; Zaninetta, G.; Zucco, F.; Bagnasco, M.; Lanata, L.; et al. Episodic (breakthrough) pain prevalence in a population of cancer pain patients. Comparison of clinical diagnoses with the QUDEI–Italian questionnaire for intense episodic pain. J. Pain Symptom Manag. 2012, 43, 833–841. [Google Scholar] [CrossRef]
- Serlin, R.C.; Mendoza, T.R.; Nakamura, Y.; Edwards, K.R.; Cleeland, C.S. When Is Cancer Pain Mild, Moderate or Severe—Grading Pain Severity by Its Interference with Function. Pain 1995, 61, 277–284. [Google Scholar] [CrossRef]
- Oldenmenger, W.H.; de Raaf, P.J.; de Klerk, C.; van der Rijt, C.C. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: A systematic review. J. Pain Symptom Manag. 2013, 45, 1083–1093. [Google Scholar] [CrossRef]
- Lohre, E.T.; Klepstad, P.; Bennett, M.I.; Brunelli, C.; Caraceni, A.; Fainsinger, R.L.; Knudsen, A.K.; Mercadante, S.; Sjogren, P.; Kaasa, S.; et al. From “Breakthrough” to “Episodic” Cancer Pain? A European Association for Palliative Care Research Network Expert Delphi Survey Toward a Common Terminology and Classification of Transient Cancer Pain Exacerbations. J. Pain Symptom Manag. 2016, 51, 1013–1019. [Google Scholar] [CrossRef]
- Klepstad, P.; Thronaes, M.; Lohre, E.T. Breakthrough pain is not a fixed fraction of constant cancer pain. Eur. J. Pain 2020, 24, 999–1000. [Google Scholar] [CrossRef]
- Caraceni, A.; Portenoy, R.K. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. Pain 1999, 82, 263–274. [Google Scholar] [CrossRef]
- Caraceni, A.; Martini, C.; Zecca, E.; Portenoy, R.K.; Ashby, M.A.; Hawson, G.; Jackson, K.A.; Lickiss, N.; Muirden, N.; Pisasale, M.; et al. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat. Med. 2004, 18, 177–183. [Google Scholar] [CrossRef]
- Lohre, E.T.; Hjermstad, M.J.; Brunelli, C.; Knudsen, A.K.; Kaasa, S.; Klepstad, P. Pain Intensity Factors Changing Breakthrough Pain Prevalence in Patients with Advanced Cancer: A Secondary Analysis of a Cross-Sectional Observational International Study. Pain Ther. 2018, 7, 193–203. [Google Scholar] [CrossRef]
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D.; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef]
- Mercadante, S.; Valle, A.; Porzio, G.; Aielli, F.; Adile, C.; Ficorella, C.; Ranieri, M.; Giarratano, A.; Casuccio, A. Relationship between background cancer pain, breakthrough pain, and analgesic treatment: A preliminary study for a better interpretation of epidemiological and clinical studies. Curr. Med. Res. Opin. 2013, 29, 667–671. [Google Scholar] [CrossRef]
- Mercadante, S.; Marchetti, P.; Cuomo, A.; Caraceni, A.; Mediati, R.D.; Vellucci, R.; Mammucari, M.; Natoli, S.; Lazzari, M.; Dauri, M.; et al. Factors Influencing the Clinical Presentation of Breakthrough Pain in Cancer Patients. Cancers 2018, 10, 175. [Google Scholar] [CrossRef]
- Brzakala, J.; Leppert, W. The role of rapid onset fentanyl products in the management of breakthrough pain in cancer patients. Pharmacol. Rep. 2019, 71, 438–442. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Talari, K.; Goyal, M. Retrospective studies—Utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.G.; Phillips, M.R. Secondary analysis of existing data: Opportunities and implementation. Shanghai Arch. Psychiatry 2014, 26, 371–375. [Google Scholar] [CrossRef]
- Webber, K.; Davies, A.N.; Zeppetella, G.; Cowie, M.R. Development and validation of the breakthrough pain assessment tool (BAT) in cancer patients. J. Pain Symptom Manag. 2014, 48, 619–631. [Google Scholar] [CrossRef]
- Fainsinger, R.L.; Nekolaichuk, C.L.; Lawlor, P.G.; Neumann, C.M.; Hanson, J.; Vigano, A. A multicenter study of the revised Edmonton Staging System for classifying cancer pain in advanced cancer patients. J. Pain Symptom Manag. 2005, 29, 224–237. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J. Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef]
- Hannon, B.; Dyck, M.; Pope, A.; Swami, N.; Banerjee, S.; Mak, E.; Bryson, J.; Rodin, G.; Ridley, J.; Lo, C.; et al. Modified Edmonton Symptom Assessment System including constipation and sleep: Validation in outpatients with cancer. J. Pain Symptom Manag. 2015, 49, 945–952. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Kaasa, S.; Caraceni, A.; Loge, J.H.; Pedersen, T.; Haugen, D.F.; Aass, N.; European Palliative Care Research, C. Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support. Palliat. Care 2016, 6, 344–352. [Google Scholar] [CrossRef]
- Davies, A.; Dickman, A.; Farquhar-Smith, P.; Webber, K.; Zeppetella, J. Letter to the Editor re ’Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer (BMJ Support Palliat Care 2016;6:344-52)’. BMJ Support. Palliat. Care 2017, 7, 264. [Google Scholar] [CrossRef]
- Caraceni, A.; Shkodra, M. Cancer Pain Assessment and Classification. Cancers 2019, 11, 510. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Payne, D.; Jacobsen, P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain 1999, 81, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Buchanan, A.; Zeppetella, G.; Porta-Sales, J.; Likar, R.; Weismayr, W.; Slama, O.; Korhonen, T.; Filbet, M.; Poulain, P.; et al. Breakthrough cancer pain: An observational study of 1000 European oncology patients. J. Pain Symptom Manag. 2013, 46, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Lazzari, M.; Reale, C.; Cuomo, A.; Fusco, F.; Marchetti, P.; Mediati, R.D.; Chiurazzi, B.; Ciuffedra, L.; Caraceni, A.; et al. Italian Oncological Pain Survey (IOPS): A multicentre Italian study of breakthrough pain performed in different settings. Clin. J. Pain 2015, 31, 214–221. [Google Scholar] [CrossRef]
- Zajaczkowska, R.; Kocot-Kepska, M.; Leppert, W.; Wordliczek, J. Bone Pain in Cancer Patients: Mechanisms and Current Treatment. Int. J. Mol. Sci. 2019, 20, 6047. [Google Scholar] [CrossRef]
- Mercadante, S.; Adile, C.; Masedu, F.; Valenti, M.; Aielli, F. Breakthrough Cancer Pain in Patients With Abdominal Visceral Cancer Pain. J. Pain Symptom Manag. 2019, 57, 966–970. [Google Scholar] [CrossRef]
- Edwards, H.L.; Mulvey, M.R.; Bennett, M.I. Cancer-Related Neuropathic Pain. Cancers 2019, 11, 373. [Google Scholar] [CrossRef]
- Hagen, N.A.; Stiles, C.; Nekolaichuk, C.; Biondo, P.; Carlson, L.E.; Fisher, K.; Fainsinger, R. The Alberta Breakthrough Pain Assessment Tool for cancer patients: A validation study using a delphi process and patient think-aloud interviews. J. Pain Symptom Manag. 2008, 35, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Bedard, G.; Davies, A.; McDonald, R.; Hawley, P.; Buchanan, A.; Popovic, M.; Wong, E.; Chow, E. Breakthrough cancer pain: A comparison of surveys with European and Canadian patients. Support. Care Cancer 2015, 23, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Batistaki, C.; Graczyk, M.; Janecki, M.; Lewandowska, A.A.; Moutinho, R.; Vagdatli, K. Relationship between breakthrough cancer pain, background cancer pain and analgesic treatment—Case series and review of the literature. Drugs Context 2022, 11, 2022-9-4. [Google Scholar] [CrossRef] [PubMed]
- Janknegt, R.; van den Beuken, M.; Schiere, S.; Uberall, M.; Knaggs, R.; Hanley, J.; Thronaes, M. Rapid acting fentanyl formulations in breakthrough pain in cancer. Drug selection by means of the System of Objectified Judgement Analysis. Eur. J. Hosp. Pharm. 2018, 25, e2. [Google Scholar] [CrossRef]
| Characteristics | Sample | Percentage |
|---|---|---|
| Gender, n (%) | ||
| Female | 43 | 32.8 |
| Male | 88 | 67.2 |
| Age, years, mean (SD 1) | 70 (13) | |
| Cancer diagnosis, n (%) | ||
| Gastrointestinal | 59 | 45.0 |
| Urological | 33 | 25.2 |
| Breast | 10 | 7.6 |
| Lung | 2 | 1.5 |
| Head/Neck | 8 | 6.1 |
| Others | 18 | 13.7 |
| Missing Metastatic status, n (%) | 1 | 0.8 |
| Metastases present | 131 | 100 |
| Skeletal metastases | 55 | 42.0 |
| Comorbidity 2, n (%) | ||
| Yes | 95 | 72.5 |
| No | 36 | 27.5 |
| WHO performance status, n (%) | ||
| WHO 1–2 | 64 | 48.9 |
| WHO 3–4 | 67 | 51.1 |
| Care trajectory, n (%) | ||
| Palliative care | 72 | 55.0 |
| Integrated oncology and palliative care | 58 | 44.3 |
| Missing | 1 | 0.8 |
| Pain flares, n (%) | ||
| Pain flares present | 90 | 68.7 |
| No pain flares | 41 | 31.3 |
| Absolute Peak Size | n | Mean | SD 1 |
|---|---|---|---|
| No pain spikes | 41 | 1.93 | 2.3 |
| Absolute pain spike intensity 1 | 30 | 1.43 | 2.5 |
| Absolute pain spike intensity 2 | 17 | 1.18 | 2.5 |
| Absolute pain spike intensity 3 | 17 | 0.94 | 2.3 |
| Absolute pain spikes intensity ≥4 | 26 | 0.08 | 1.9 |
| Peak Ratio Group | n | Mean | SD 1 |
|---|---|---|---|
| Relative pain spike intensity <0.49 | 37 | 1.92 | 2.6 |
| Relative pain spike intensity 0.5–0.99 | 17 | 1.24 | 1.5 |
| Relative pain spike intensity 1.0–1.49 | 14 | 0.00 | 1.8 |
| Relative pain spike intensity ≥1.5 | 14 | −0.71 2 | 2.1 |
| Group | n | Transmucosal Fentanyl, n (%) | Parenteral Opioids, n (%) | Peroral Opioids, n (%) |
|---|---|---|---|---|
| Patients with absolute pain spike intensity ≥4 | 26 | 0 | 12 (46.2%) | 11 (42.3%) |
| The remaining group of patients with pain flares | 64 | 2 (3.1%) | 23 (35.9%) | 38 (59.4%) |
| Patients with no pain flares | 41 | 1 (2.4%) | 14 (34.1%) | 22 (53.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Løhre, E.T.; Jakobsen, G.; Solheim, T.S.; Klepstad, P.; Thronæs, M. Breakthrough and Episodic Cancer Pain from a Palliative Care Perspective. Curr. Oncol. 2023, 30, 10249-10259. https://doi.org/10.3390/curroncol30120746
Løhre ET, Jakobsen G, Solheim TS, Klepstad P, Thronæs M. Breakthrough and Episodic Cancer Pain from a Palliative Care Perspective. Current Oncology. 2023; 30(12):10249-10259. https://doi.org/10.3390/curroncol30120746
Chicago/Turabian StyleLøhre, Erik Torbjørn, Gunnhild Jakobsen, Tora Skeidsvoll Solheim, Pål Klepstad, and Morten Thronæs. 2023. "Breakthrough and Episodic Cancer Pain from a Palliative Care Perspective" Current Oncology 30, no. 12: 10249-10259. https://doi.org/10.3390/curroncol30120746