Immune Checkpoint Glycoproteins Have Polymorphism: Are Monoclonal Antibodies Too Specific?
Abstract
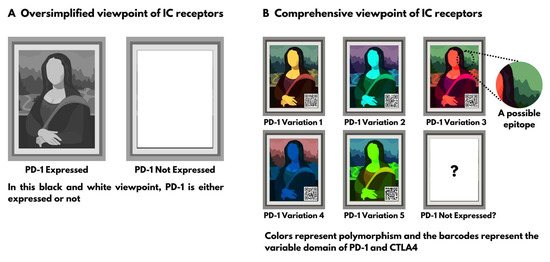
:1. Introduction
We are each unique, and so is our immune system.
2. Section 1: Evidence on Polymorphism of Immune Checkpoint Receptors
2.1. Genotype Polymorphism
2.2. End Product (Phenotype) Polymorphism
- 1.
- When an mAb assay fails to stain a tumor specimen from a patient, it does NOT mean that the antigen is absent; it can mean that the specific epitope for this clone of staining mAb is absent, and the assay is unable to detect the variant that this patient is expressing. One assay of a single clone of mAb is not sensitive enough to rule out the expression of these molecules due to their polymorphism.
- 2.
- If a patient specimen is stained positive for the presence of the antigen, it does NOT mean that the treatment mAb will also recognize the antigen. Staining mAbs are designed to attach to formaldehyde fixated glycoproteins at room temperature and are completely different from those designed to be injected and attached to the natural form of the glycoprotein in physiological body state. According to Brown et al. [29], treatment mAbs have a polymorphism in their affinity to different epitopes.
2.3. Further Evidence
3. Section 2: Why Is This Polymorphism Overlooked?
- a.
- Laboratory animals are inbred and highly identical (isogenic or semi-isogenic). Individual differences in the PD-1, PD-L1 and CTLA4 are less expected in generationally inbred animals.
- b.
- Tumors in laboratory animals are often created by injection of a well-established cultured cell line; polymorphism is less expected in these cell cultures.
- c.
- Laboratory animals are kept in sterile conditions. Their immune system is therefore naïve, untrained and can act differently from humans who have been in contact with myriads of pathogenic and non-pathogenic germs since birth.
4. Section 3: Authors’ Suggestions
4.1. The Monospecific Nature of mAbs Is a Double-Edged Sword
4.2. First Solution: Reduce Specificity and Increase Sensitivity
4.2.1. Polyclonal Antibodies and Recombinant Polyclonal Antibodies
4.2.2. Monoclonal “Cocktails” or Oligoclonal Antibodies
4.3. Second Solution: Increase Specificity to the Individual Level and Tailor It
Author Contributions
Funding
Conflicts of Interest
References
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubat, T.; Yagita, H.; Honjo, T. Expression of the PD-1 Antigen on the Surface of Stimulated Mouse T and B Lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korman, A.J.; Peggs, K.S.; Allison, J.P. Checkpoint Blockade in Cancer Immunotherapy. Adv. Immunol. 2006, 90, 297–339. [Google Scholar] [PubMed] [Green Version]
- Gravbrot, N.; Gilbert-Gard, K.; Mehta, P.; Ghotmi, Y.; Banerjee, M.; Mazis, C.; Sundararajan, S. Therapeutic Monoclonal Antibodies Targeting Immune Checkpoints for the Treatment of Solid Tumors. Antibodies 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zak, K.M.; Grudnik, P.; Magiera, K.; Dömling, A.; Dubin, G.; Holak, T.A. Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2. Structure 2017, 25, 1163–1174. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers 2021, 13, 131. [Google Scholar] [CrossRef]
- Rhea, L.P.; Aragon-Ching, J.B. Advances and Controversies with Checkpoint Inhibitors in Bladder Cancer. Clin. Med. Insights Oncol. 2021, 15, 11795549211044963. [Google Scholar] [CrossRef]
- National Cancer Institute. FDA Alters Approved Use of Two Checkpoint Inhibitors for Bladder Cancer. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2018/bladder-cancer-checkpoint-inhibitor-change (accessed on 20 December 2022).
- Rajewsky, K. The Advent and Rise of Monoclonal Antibodies. Nature 2019, 575, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Ascoli, C.A.; Aggeler, B. Overlooked Benefits of Using Polyclonal Antibodies. Biotechniques 2018, 65, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Haurum, J.S. Recombinant Polyclonal Antibodies: The next Generation of Antibody Therapeutics? Drug Discov. Today 2006, 11, 655–660. [Google Scholar] [CrossRef]
- Larbouret, C.; Gros, L.; Pèlegrin, A.; Chardès, T. Improving Biologics’ Effectiveness in Clinical Oncology: From the Combination of Two Monoclonal Antibodies to Oligoclonal Antibody Mixtures. Cancers 2021, 13, 4620. [Google Scholar] [CrossRef]
- Lafage-Pochitaloff, M.; Costello, R.; Couez, D.; Simonetti, J.; Mannoni, P.; Mawas, C.; Olive, D. Human CD28 and CTLA-4 Ig Superfamily Genes Are Located on Chromosome 2 at Bands Q33-Q34. Immunogenetics 1990, 31, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Taniwaki, M.; Ishida, Y.; Kawaichi, M.; Honjo, T. Structure and Chromosomal Localization of the Human PD-1 Gene (PDCD1). Genomics 1994, 23, 704–706. [Google Scholar] [CrossRef]
- Fabrizio, F.P.; Trombetta, D.; Rossi, A.; Sparaneo, A.; Castellana, S.; Muscarella, L.A. Gene Code CD274/PD-L1: From Molecular Basis toward Cancer Immunotherapy. Ther. Adv. Med. Oncol. 2018, 10, 1758835918815598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, M.; Karami, S.; Sarabandi, S.; Moazeni-Roodi, A.; Małecki, A.; Ghavami, S.; Wiechec, E. Association between PD-1 and PD-L1 Polymorphisms and the Risk of Cancer: A Meta-Analysis of Case-Control Studies. Cancers 2019, 11, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simone, R.; Saverino, D. The Soluble CTLA-4 Receptor and Its Emerging Role in Autoimmune Diseases. Curr. Immunol. Rev. 2009, 5, 54–68. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Khoramshahi, V.; Azani, A.; Soltaninejad, E.; Aslani, S.; Zamani, M.R.; Zal, M.; Nesaei, A.; Hosseini, S.M. PD-1 and Cancer: Molecular Mechanisms and Polymorphisms. Immunogenetics 2018, 70, 73–86. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, H.-X.; Yao, Y.; Bian, Z.-H.; Zheng, Y.-J.; Li, L.; Moutsopoulos, H.M.; Gershwin, M.E.; Lian, Z.-X. Immune Checkpoint Molecules. Possible Future Therapeutic Implications in Autoimmune Diseases. J. Autoimmun. 2019, 104, 102333. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). Reference SNP (rs) Report. Homo sapiens: rs2227981. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2227981 (accessed on 20 December 2022).
- Hultén, M.A. On the Origin of Crossover Interference: A Chromosome Oscillatory Movement (COM) Model. Mol. Cytogenet. 2011, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Eckstein, M.; Erben, P.; Kriegmair, M.C.; Worst, T.S.; Weiß, C.-A.; Wirtz, R.M.; Wach, S.; Stoehr, R.; Sikic, D.; Geppert, C.I.; et al. Performance of the Food and Drug Administration/EMA-Approved Programmed Cell Death Ligand-1 Assays in Urothelial Carcinoma with Emphasis on Therapy Stratification for First-Line Use of Atezolizumab and Pembrolizumab. Eur. J. Cancer 2019, 106, 234–243. [Google Scholar] [CrossRef]
- Munari, E.; Querzoli, G.; Brunelli, M.; Marconi, M.; Sommaggio, M.; Cocchi, M.A.; Martignoni, G.; Netto, G.J.; Caliò, A.; Quatrini, L.; et al. Comparison of Three Validated PD-L1 Immunohistochemical Assays in Urothelial Carcinoma of the Bladder: Interchangeability and Issues Related to Patient Selection. Front. Immunol. 2022, 13, 954910. [Google Scholar] [CrossRef]
- Zajac, M.; Scott, M.; Ratcliffe, M.; Scorer, P.; Barker, C.; Al-Masri, H.; Rebelatto, M.C.; Walker, J. Concordance among Four Commercially Available, Validated Programmed Cell Death Ligand-1 Assays in Urothelial Carcinoma. Diagn. Pathol. 2019, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuman, T.; London, M.; Kania-Almog, J.; Litvin, A.; Zohar, Y.; Fridel, L.; Sandbank, J.; Barshak, I.; Vainer, G.W. A Harmonization Study for the Use of 22C3 PD-L1 Immunohistochemical Staining on Ventana’s Platform. J. Thorac. Oncol. 2016, 11, 1863–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwamborn, K.; Ammann, J.U.; Knüchel, R.; Hartmann, A.; Baretton, G.; Lasitschka, F.; Schirmacher, P.; Braunschweig, T.; Tauber, R.; Erlmeier, F.; et al. Multicentric Analytical Comparability Study of Programmed Death-Ligand 1 Expression on Tumor-Infiltrating Immune Cells and Tumor Cells in Urothelial Bladder Cancer Using Four Clinically Developed Immunohistochemistry Assays. Virchows Arch. 2019, 475, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.L.; Hsiao, Y.J.; Cooper, W.A.; Choi, Y.L.; Avilés-Salas, A.; Chou, T.Y.; Coudry, R.; Raskin, G.A.; Fox, S.B.; Huang, C.C.; et al. The Ring Study: An International Comparison of PD-L1 Diagnostic Assays and Their Interpretation in Non-Small Cell Lung Cancer, Head and Neck Squamous Cell Cancer and Urothelial Cancer. Pathology 2022, 55, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Kintsler, S.; Cassataro, M.A.; Drosch, M.; Holenya, P.; Knuechel, R.; Braunschweig, T. Expression of Programmed Death Ligand (PD-L1) in Different Tumors. Comparison of Several Current Available Antibody Clones and Antibody Profiling. Ann. Diagn. Pathol. 2019, 41, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, S.J.; Chen, J.; Lu, S.X.; Fan, X.J.; Tong, J.H.M.; Chow, C.; Tin, E.K.Y.; Chan, S.L.; Chong, C.C.N.; et al. A Comparability Study of Immunohistochemical Assays for PD-L1 Expression in Hepatocellular Carcinoma. Mod. Pathol. 2019, 32, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Bedinger, D.; Lilov, A.; Rathanaswami, P.; Vásquez, M.; Durand, S.; Wallace-Moyer, I.; Zhong, L.; Nett, J.H.; Burnina, I.; et al. Assessing the Binding Properties of the Anti-PD-1 Antibody Landscape Using Label-Free Biosensors. PLoS ONE 2020, 15, e0229206. [Google Scholar] [CrossRef] [Green Version]
- Lefranc, M.P. IMGT Unique Numbering. In Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013; pp. 952–959. [Google Scholar]
- Zak, K.M.; Kitel, R.; Przetocka, S.; Golik, P.; Guzik, K.; Musielak, B.; Dömling, A.; Dubin, G.; Holak, T.A. Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1. Structure 2015, 23, 2341–2348. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Lin, D.Y.; Tanaka, Y.; Iwasaki, M.; Gittis, A.G.; Su, H.-P.; Mikami, B.; Okazaki, T.; Honjo, T.; Minato, N.; Garboczi, D.N. The PD-1/PD-L1 Complex Resembles the Antigen-Binding Fv Domains of Antibodies and T Cell Receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 3011–3016. [Google Scholar] [CrossRef] [Green Version]
- Research and Markets. In Global Immune Checkpoint Inhibitors Market Outlook 2022; Research and Markets, The world’s largest Market Research Store; RNCOS E-Services Private Limited: Dubai, UAE, 2020; 130p.
- Newcombe, C.; Newcombe, A.R. Antibody Production: Polyclonal-Derived Biotherapeutics. J. Chromatogr. B 2007, 848, 2–7. [Google Scholar] [CrossRef]
- Encarnação, J.C.; Barta, P.; Fornstedt, T.; Andersson, K. Impact of Assay Temperature on Antibody Binding Characteristics in Living Cells: A Case Study. Biomed. Rep. 2017, 7, 400–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devanaboyina, S.C.; Lynch, S.M.; Ober, R.J.; Ram, S.; Kim, D.; Puig-Canto, A.; Breen, S.; Kasturirangan, S.; Fowler, S.; Peng, L.; et al. The Effect of PH Dependence of Antibody-Antigen Interactions on Subcellular Trafficking Dynamics. MAbs 2013, 5, 851–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Coljee, V.W.; Maynard, J.A. Back to the Future: Recombinant Polyclonal Antibody Therapeutics. Curr. Opin. Chem. Eng. 2013, 2, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Yadollahvandmiandoab, R.; Jalalizadeh, M.; Buosi, K.; Garcia-Perdomo, H.A.; Reis, L.O. Immunogenic Cell Death Role in Urothelial Cancer Therapy. Curr. Oncol. 2022, 29, 6700–6713. [Google Scholar] [CrossRef]
- Bai, X.F.; Liu, J.; Li, O.; Zheng, P.; Liu, Y. Antigenic Drift as a Mechanism for Tumor Evasion of Destruction by Cytolytic T Lymphocytes. J. Clin. Investig. 2003, 111, 1487–1496. [Google Scholar] [CrossRef]
- Pirch, J.H.; Peterson, S.L. Event-Related Slow Potentials and Activity of Singly Neurons in Rat Frontal Cortex. Int. J. Neurosci. 1981, 15, 141–146. [Google Scholar] [CrossRef]
- Zajicek, J.; Wing, M.; Skepper, J.; Compston, A. Human Oligodendrocytes Are Not Sensitive to Complement. A Study of CD59 Expression in the Human Central Nervous System. Lab. Investig. 1995, 73, 128–138. [Google Scholar]
- Carvalho, S.; Levi-Schaffer, F.; Sela, M.; Yarden, Y. Immunotherapy of Cancer: From Monoclonal to Oligoclonal Cocktails of Anti-Cancer Antibodies: IUPHAR Review 18. Br. J. Pharmacol. 2016, 173, 1407–1424. [Google Scholar] [CrossRef] [Green Version]
- Rozsíval, P.; Sercl, M. Computerized Tomography—Principles and Advantages for Diagnosis of Orbital Diseases. Cesk. Oftalmol. 1982, 38, 297–302. [Google Scholar]
- Corti, D.; Kearns, J.D. Promises and Pitfalls for Recombinant Oligoclonal Antibodies-Based Therapeutics in Cancer and Infectious Disease. Curr. Opin. Immunol. 2016, 40, 51–61. [Google Scholar] [CrossRef]
- The Nobel Prize. Prize Announcement—Nobel Prize Outreach AB 2023. Available online: https://www.nobelprize.org/prizes/medicine/2018/prize-announcement (accessed on 20 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalalizadeh, M.; Yadollahvandmiandoab, R.; Reis, L.O. Immune Checkpoint Glycoproteins Have Polymorphism: Are Monoclonal Antibodies Too Specific? Curr. Oncol. 2023, 30, 1267-1274. https://doi.org/10.3390/curroncol30010098
Jalalizadeh M, Yadollahvandmiandoab R, Reis LO. Immune Checkpoint Glycoproteins Have Polymorphism: Are Monoclonal Antibodies Too Specific? Current Oncology. 2023; 30(1):1267-1274. https://doi.org/10.3390/curroncol30010098
Chicago/Turabian StyleJalalizadeh, Mehrsa, Reza Yadollahvandmiandoab, and Leonardo Oliveira Reis. 2023. "Immune Checkpoint Glycoproteins Have Polymorphism: Are Monoclonal Antibodies Too Specific?" Current Oncology 30, no. 1: 1267-1274. https://doi.org/10.3390/curroncol30010098