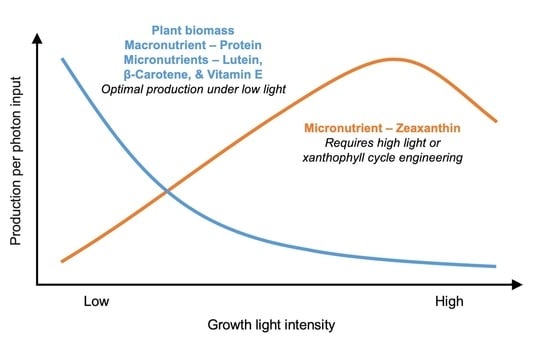
Lemna as a Sustainable, Highly Nutritious Crop: Nutrient Production in Different Light Environments
Abstract
:1. Introduction
1.1. Dietary Carotenoids and Human Health
1.2. Floating Plants with Exceptional Nutritional Quality and Sustainable Production
2. Materials and Methods
2.1. Plant Growth and Assessment of Nutrient Content
2.2. Light-Use Efficiency
2.3. Statistical Analysis
3. Results
3.1. The Impact of Reference Basis on the Assessment of Photosynthetic Performance
3.2. Growth Rate, Dry Biomass per Area, and Light-Use Efficiency of Biomass Production as a Function of Growth PFD
3.3. Lutein, β-Carotene, and α-Tocopherol Production on a Dry Biomass Basis and per Photons Received as a Function of Growth PFD
3.4. Zeaxanthin Production on a Dry Biomass Basis and per Photons Received as a Function of Growth PFD
3.5. Variations in Carotenoids and Vitamin E Dynamics
3.6. Protein Production on a Dry Biomass Basis and per Photons Received as a Function of Growth PFD
4. Discussion and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCartney, L.; Lefsrud, M. Protected Agriculture in Extreme Environments: A Review of Controlled Environment Agriculture in Tropical, Arid, Polar, and Urban Locations. Appl. Eng. Agric. 2018, 34, 455–473. [Google Scholar] [CrossRef]
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.-J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled Environment Food Production for Urban Agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Cohu, C.M.; Lombardi, E.; Adams, W.W., III; Demmig-Adams, B. Increased Nutritional Quality of Plants for Long-Duration Spaceflight Missions through Choice of Plant Variety and Manipulation of Growth Conditions. Acta Astronaut. 2014, 94, 799–806. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Adams, W.W., III; Escobar, C.M.; Demmig-Adams, B. Conquering Space with Crops That Produce Ample Oxygen and Antioxidants. Oxygen 2022, 2, 211–226. [Google Scholar] [CrossRef]
- Popkin, B.M. The Nutrition Transition: An Overview of World Patterns of Change. Nutr. Rev. 2004, 62, S140–S143. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed Foods and the Nutrition Transition: Global, Regional and National Trends, Food Systems Transformations and Political Economy Drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 Threatens Human Nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; López-Pozo, M.; Polutchko, S.K.; Fourounjian, P.; Stewart, J.J.; Zenir, M.C.; Adams, W.W., III. Growth and Nutritional Quality of Lemnaceae Viewed Comparatively in an Ecological and Evolutionary Context. Plants 2022, 11, 145. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of Chronic Inflammatory Disease: Universal and Tissue-Specific Concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Ashraf-Ganjouei, A.; Moradi, K.; Bagheri, S.; Aarabi, M.H. The Association between Systemic Inflammation and Cognitive Performance in Healthy Adults. J. Neuroimmunol. 2020, 345, 577272. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; Beekman, A.T.F.; De Jonge, P.; Penninx, B. Anxiety Disorders and Inflammation in a Large Adult Cohort. Transl. Psychiatry 2013, 3, e249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavich, G.M.; Irwin, M.R. From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danesh, J.; Whincup, P.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Gallimore, J.R.; Pepys, M.B. Low Grade Inflammation and Coronary Heart Disease: Prospective Study and Updated Meta-Analyses. BMJ 2000, 321, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Enichen, E.; Adams, R.B.; Demmig-Adams, B. Physical Activity as an Adjunct Treatment for People Living with HIV? Am. J. Lifestyle Med. 2022, 155982762210782. [Google Scholar] [CrossRef]
- Wu, D.; Yang, X.O. TH17 Responses in Cytokine Storm of COVID-19: An Emerging Target of JAK2 Inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Enichen, E.; Harvey, C.; Demmig-Adams, B. COVID-19 Spotlights Connections between Disease and Multiple Lifestyle Factors. Am. J. Lifestyle Med. 2022, 15598276221123005. [Google Scholar] [CrossRef]
- Haran, J.P.; Bradley, E.; Zeamer, A.L.; Cincotta, L.; Salive, M.-C.; Dutta, P.; Mutaawe, S.; Anya, O.; Meza-Segura, M.; Moormann, A.M.; et al. Inflammation-Type Dysbiosis of the Oral Microbiome Associates with the Duration of COVID-19 Symptoms and Long COVID. JCI Insight 2021, 6, e152346. [Google Scholar] [CrossRef]
- Evans, R.A.; Leavy, O.C.; Richardson, M.; Elneima, O.; McCauley, H.J.C.; Shikotra, A.; Singapuri, A.; Sereno, M.; Saunders, R.M.; Harris, V.C.; et al. Clinical Characteristics with Inflammation Profiling of Long COVID and Association with 1-Year Recovery Following Hospitalisation in the UK: A Prospective Observational Study. Lancet Respir. Med. 2022, 10, 761–775. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Glime, G.N.; Demmig-Adams, B. Synergistic Action of Membrane-Bound and Water-Soluble Antioxidants in Neuroprotection. Molecules 2021, 26, 5385. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Polutchko, S.K.; Adams, W.W., III. Structure-Function-Environment Relationship of the Isomers Zeaxanthin and Lutein. Photochem 2022, 2, 308–325. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The Role of Carotenoids in the Prevention of Human Pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and Protection of Phospholipids in Solution or in Liposomes against Oxidation by Peroxyl Radicals: Relationship between Carotenoid Structure and Protective Ability. Biochim. Biophys. Acta BBA—Gen. Subj. 1997, 1336, 575–586. [Google Scholar] [CrossRef]
- Wrona, M.; Korytowski, W.; Różanowska, M.; Sarna, T.; Truscott, T.G. Cooperation of Antioxidants in Protection against Photosensitized Oxidation. Free Radic. Biol. Med. 2003, 35, 1319–1329. [Google Scholar] [CrossRef]
- Wrona, M.; Różanowska, M.; Sarna, T. Zeaxanthin in Combination with Ascorbic Acid or α-Tocopherol Protects ARPE-19 Cells against Photosensitized Peroxidation of Lipids. Free Radic. Biol. Med. 2004, 36, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Zwart, S.R. Nutritional Biochemistry of Spaceflight. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 46, pp. 87–130. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; Ostan, R.; Cevenini, E.; Gonos, E.S.; Franceschi, C.; et al. Vitamin E-Gene Interactions in Aging and Inflammatory Age-Related Diseases: Implications for Treatment. A Systematic Review. Ageing Res. Rev. 2014, 14, 81–101. [Google Scholar] [CrossRef] [Green Version]
- Peh, H.Y.; Tan, W.D.; Liao, W.; Wong, W.F. Vitamin E Therapy beyond Cancer: Tocopherol versus Tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Zareba, M.; Widomska, J.; Burke, J.M.; Subczynski, W.K. Nitroxide Free Radicals Protect Macular Carotenoids against Chemical Destruction (Bleaching) during Lipid Peroxidation. Free Radic. Biol. Med. 2016, 101, 446–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, L.D.; Bandara, S.; Parmar, V.M.; Srinivasagan, R.; Khadka, N.; Golczak, M.; Kiser, P.D.; von Lintig, J. The Human Mitochondrial Enzyme BCO2 Exhibits Catalytic Activity toward Carotenoids and Apocarotenoids. J. Biol. Chem. 2020, 295, 15553–15565. [Google Scholar] [CrossRef] [PubMed]
- Sayo, T.; Sugiyama, Y.; Inoue, S. Lutein, a Nonprovitamin A, Activates the Retinoic Acid Receptor to Induce HAS3-Dependent Hyaluronan Synthesis in Keratinocytes. Biosci. Biotechnol. Biochem. 2013, 77, 1282–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.; Dergunov, S.A.; Zhang, W.; Gentleman, S.; Redmond, T.M.; Pinkhassik, E.; Poliakov, E. Xanthophylls Modulate Palmitoylation of Mammalian β-Carotene Oxygenase 2. Antioxidants 2021, 10, 413. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-Inflammatory Cytokines and Adipose Tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Trayhurn, P.; Beattie, J.H. Physiological Role of Adipose Tissue: White Adipose Tissue as an Endocrine and Secretory Organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zheng, M.; Cai, D.; Xie, J.; Jin, Z.; Liu, H.; Liu, J. Zeaxanthin Promotes Mitochondrial Biogenesis and Adipocyte Browning via AMPKα1 Activation. Food Funct. 2019, 10, 2221–2233. [Google Scholar] [CrossRef]
- Xie, J.; Liu, M.; Liu, H.; Jin, Z.; Guan, F.; Ge, S.; Yan, J.; Zheng, M.; Cai, D.; Liu, J. Zeaxanthin Ameliorates Obesity by Activating the β3-Adrenergic Receptor to Stimulate Inguinal Fat Thermogenesis and Modulating the Gut Microbiota. Food Funct. 2021, 12, 12734–12750. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, M.; Liu, H.; Xie, J.; Yan, J.; Hou, X.; Liu, J. Zeaxanthin Promotes Browning by Enhancing Mitochondrial Biogenesis through the PKA Pathway in 3T3-L1 Adipocytes. Food Funct. 2021, 12, 6283–6293. [Google Scholar] [CrossRef]
- El Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Food Ingredients Involved in White-to-Brown Adipose Tissue Conversion and in Calorie Burning. Front. Physiol. 2019, 9, 1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schalch, W.; Landrum, J.T.; Bone, R.A. The eye. In Carotenoids. Nutrition and Health; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; Volume 5, pp. 301–334. [Google Scholar] [CrossRef]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.-Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging Lutein and Zeaxanthin in the Human Retina with Confocal Resonance Raman Microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic Mechanisms of the Protective Role of Lutein and Zeaxanthin in the Eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richer, S.P.; Stiles, W.; Graham-Hoffman, K.; Levin, M.; Ruskin, D.; Wrobel, J.; Park, D.-W.; Thomas, C. Randomized, Double-Blind, Placebo-Controlled Study of Zeaxanthin and Visual Function in Patients with Atrophic Age-Related Macular Degeneration: The Zeaxanthin and Visual Function Study (ZVF) FDA IND# 78, 973. Optom.-J. Am. Optom. Assoc. 2011, 82, 667–680. [Google Scholar] [CrossRef]
- Zaragoza-Huesca, D.; Martínez-Cortés, C.; Banegas-Luna, A.J.; Pérez-Garrido, A.; Vegara-Meseguer, J.M.; Peñas-Martínez, J.; Rodenas, M.C.; Espín, S.; Pérez-Sánchez, H.; Martínez-Martínez, I. Identification of Kukoamine A, Zeaxanthin, and Clexane as New Furin Inhibitors. Int. J. Mol. Sci. 2022, 23, 2796. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; Rohde, C.; et al. TMPRSS2 and Furin Are Both Essential for Proteolytic Activation of SARS-CoV-2 in Human Airway Cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Overview of Diet-Gene Interaction and the Example of Xanthophylls. Adv. Exp. Med. Biol. 2010, 698, 17–26. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The Body of Evidence to Support a Protective Role for Lutein and Zeaxanthin in Delaying Chronic Disease. Overview. J. Nutr. 2002, 132, 518S–524S. [Google Scholar] [CrossRef] [Green Version]
- Ribaya-Mercado, J.D.; Blumberg, J.B. Lutein and Zeaxanthin and Their Potential Roles in Disease Prevention. J. Am. Coll. Nutr. 2004, 23, 567S–587S. [Google Scholar] [CrossRef]
- Bhanthumnavin, K.; McGarry, M.G. Wolffia arrhiza as a Possible Source of Inexpensive Protein. Nature 1971, 232, 495. [Google Scholar] [CrossRef] [PubMed]
- De Beukelaar, M.F.; Zeinstra, G.G.; Mes, J.J.; Fischer, A.R. Duckweed as Human Food. The Influence of Meal Context and Information on Duckweed Acceptability of Dutch Consumers. Food Qual. Prefer. 2019, 71, 76–86. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and Essential Carotenoid Micronutrients in Lemna gibba as a Function of Growth Light Intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional Value of the Duckweed Species of the Genus Wolffia (Lemnaceae) as Human Food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional Value of Duckweeds (Lemnaceae) as Human Food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Sree, K.S.; Dahse, H.-M.; Chandran, J.N.; Schneider, B.; Jahreis, G.; Appenroth, K.J. Duckweed for Human Nutrition: No Cytotoxic and No Anti-Proliferative Effects on Human Cell Lines. Plant Foods Hum. Nutr. 2019, 74, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Doherty Garcia, N.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity. Cells 2021, 10, 1481. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Tuohy, K.; von Bergen, M.; Krajmalnik-Brown, R.; Heinig, U.; Zelicha, H.; Tsaban, G.; Rinott, E.; Kaplan, A.; Aharoni, A.; et al. The Metabolomic-Gut-Clinical Axis of Mankai Plant-Derived Dietary Polyphenols. Nutrients 2021, 13, 1866. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Augsten, H.; Liebermann, B.; Feist, H. Effects of Light Quality on Amino Acid Composition of Proteins in Wolffia arrhiza (L.) Wimm. Using a Specially Modified Bradford Method. Biochem. Physiol. Pflanz. 1982, 177, 251–258. [Google Scholar] [CrossRef]
- Skillicorn, P.; Spira, W.; Journey, W. Duckweed Aquaculture: A New Aquatic Farming System for Developing Countries; The World Bank: Washington, DC, USA, 1993. [Google Scholar]
- Mohedano, R.A.; Costa, R.H.; Tavares, F.A.; Belli Filho, P. High Nutrient Removal Rate from Swine Wastes and Protein Biomass Production by Full-Scale Duckweed Ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Oron, G.; Wildschut, L.; Porath, D. Waste Water Recycling by Duckweed for Protein Production and Effluent Renovation. Water Sci. Technol. 1985, 17, 803–817. [Google Scholar] [CrossRef]
- Oron, G.; Porath, D.; Jansen, H. Performance of the Duckweed Species Lemna gibba on Municipal Wastewater for Effluent Renovation and Protein Production. Biotechnol. Bioeng. 1987, 29, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Oron, G. Duckweed Culture for Wastewater Renovation and Biomass Production. Agric. Water Manag. 1994, 26, 27–40. [Google Scholar] [CrossRef]
- Elshafai, S.; Elgohary, F.; Nasr, F.; Petervandersteen, N.; Gijzen, H. Nutrient Recovery from Domestic Wastewater Using a UASB-Duckweed Ponds System. Bioresour. Technol. 2007, 98, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 Fatty Acids and Cancer. Indoor Built Environ. 2003, 12, 405–412. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-6/Omega-3 Essential Fatty Acid Ratio and Chronic Diseases. Food Rev. Int. 2004, 20, 77–90. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio, Genetic Variation, and Cardiovascular Disease. Asia Pac. J. Clin. Nutr. 2008, 17, 131–134. [Google Scholar]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary Factors and Low-Grade Inflammation in Relation to Overweight and Obesity. Br. J. Nutr. 2011, 106, S1–S78. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 Fatty Acids and Inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Andersen, I.H.; Dons, C.; Nilsen, S.; Haugstad, M.K. Growth, Photosynthesis and Photorespiration of Lemna gibba: Response to Variations in CO2 and O2 Concentrations and Photon Flux Density. Photosynth. Res. 1985, 6, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mohedano, R.A.; Costa, R.H.R.; Filho, P.B. Effects of CO2 Concentration on Nutrient Uptake and Starch Accumulation by Duckweed Used for Wastewater Treatment and Bioethanol Production. Rev. Latinoam. Biotecnol. Ambient. Algal 2016, 7, 3. [Google Scholar] [CrossRef]
- Kittiwongwattana, C.; Thawai, C. Rhizobium lemnae Sp. Nov., a Bacterial Endophyte of Lemna aequinoctialis. Int. J. Syst. Evol. Microbiol. 2014, 64, 2455–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Haberer, G.; Gundlach, H.; Gläßer, C.; Nussbaumer, T.; Luo, M.C.; Lomsadze, A.; Borodovsky, M.; Kerstetter, R.A.; Shanklin, J. The Spirodela Polyrhiza Genome Reveals Insights into Its Neotenous Reduction Fast Growth and Aquatic Lifestyle. Nat. Commun. 2014, 5, 3311. [Google Scholar] [CrossRef] [Green Version]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Kishchenko, O.; Stepanenko, A.; Chen, G.; Wang, W.; Zhou, J.; Pan, C.; Borisjuk, N. The Dynamics of NO3− and NH4+ Uptake in Duckweed Are Coordinated with the Expression of Major Nitrogen Assimilation Genes. Plants 2021, 11, 11. [Google Scholar] [CrossRef]
- Hammouda, O.; Gaber, A.; Abdel-Hameed, M.S. Assessment of the Effectiveness of Treatment of Wastewater-Contaminated Aquatic Systems with Lemna gibba. Enzyme Microb. Technol. 1995, 17, 317–323. [Google Scholar] [CrossRef]
- Ozengin, N.; Elmaci, A. Performance of Duckweed (Lemna minor L.) on Different Types of Wastewater Treatment. J. Environ. Biol. 2007, 28, 307–314. [Google Scholar]
- Panfili, I.; Bartucca, M.L.; Del Buono, D. The Treatment of Duckweed with a Plant Biostimulant or a Safener Improves the Plant Capacity to Clean Water Polluted by Terbuthylazine. Sci. Total Environ. 2019, 646, 832–840. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Stewart, J.J.; Adams, W.W., III; Demmig-Adams, B. Photosynthesis and Foliar Vascular Adjustments to Growth Light Intensity in Summer Annual Species with Symplastic and Apoplastic Phloem Loading. J. Plant Physiol. 2021, 267, 153532. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Demmig-Adams, B. Photosynthetic Modulation in Response to Plant Activity and Environment. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 493–563. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Polutchko, S.K.; Demmig-Adams, B. Leaf Vasculature and the Upper Limit of Photosynthesis. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 27–54. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant Carbon Metabolism and Climate Change: Elevated CO2 and Temperature Impacts on Photosynthesis, Photorespiration and Respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a Model Plant System in the Genomics and Postgenomics Era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef] [PubMed]
- Sree, K.S.; Sudakaran, S.; Appenroth, K.-J. How Fast Can Angiosperms Grow? Species and Clonal Diversity of Growth Rates in the Genus Wolffia (Lemnaceae). Acta Physiol. Plant. 2015, 37, 204. [Google Scholar] [CrossRef]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.-J. Relative in Vitro Growth Rates of Duckweeds (Lemnaceae)—The Most Rapidly Growing Higher Plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein Is Needed for Efficient Chlorophyll Triplet Quenching in the Major LHCII Antenna Complex of Higher Plants and Effective Photoprotection in Vivo under Strong Light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, S.; Li, Z.; Niyogi, K.K.; Bassi, R.; Dall’Osto, L. The Arabidopsis szl1 Mutant Reveals a Critical Role of β-Carotene in Photosystem I Photoprotection. Plant Physiol. 2012, 159, 1745–1758. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet Oxygen Production in Photosystem II and Related Protection Mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M.; García-Plazaola, J.I. Beyond Non-Photochemical Fluorescence Quenching: The Overlapping Antioxidant Functions of Zeaxanthin and Tocopherols. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603. [Google Scholar] [CrossRef]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- Martindale, W.; Bowes, G. The effects of irradiance and CO2 on the activity and activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in the aquatic plant Spirodela polyrhiza. J. Exp. Bot. 1996, 47, 781–784. [Google Scholar] [CrossRef] [Green Version]
- Escobar, C.M.; Escobar, A.C.; Power, G.J.; Nabity, J.A. µG-LilyPondTM: Preliminary Design of a Floating Plant Pond for Microgravity. In Proceedings of the 50th International Conference on Environmental Systems, Lisbon, Portugal, 12–16 July 2020. [Google Scholar]
- Rinott, E.; Youngster, I.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173. [Google Scholar] [CrossRef]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The Effects of the Green-Mediterranean Diet on Cardiometabolic Health Are Linked to Gut Microbiome Modifications: A Randomized Controlled Trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Zelicha, H.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Levakov, G.; Prager, O.; Salti, M.; Yovell, Y.; Ofer, J.; et al. The Effect of a High-Polyphenol Mediterranean Diet (Green-MED) Combined with Physical Activity on Age-Related Brain Atrophy: The Dietary Intervention Randomized Controlled Trial Polyphenols Unprocessed Study (DIRECT PLUS). Am. J. Clin. Nutr. 2022, 115, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Sela, I.; Yaskolka Meir, A.; Brandis, A.; Krajmalnik-Brown, R.; Zeibich, L.; Chang, D.; Dirks, B.; Tsaban, G.; Kaplan, A.; Rinott, E.; et al. Wolffia globosa–Mankai Plant-Based Protein Contains Bioactive Vitamin B12 and Is Well Absorbed in Humans. Nutrients 2020, 12, 3067. [Google Scholar] [CrossRef]
- Tsaban, G.; Meir, A.Y.; Rinott, E.; Zelicha, H.; Kaplan, A.; Shalev, A.; Katz, A.; Rudich, A.; Tirosh, A.; Shelef, I.; et al. The Effect of Green Mediterranean Diet on Cardiometabolic Risk; a Randomised Controlled Trial. Heart 2020, 107, 1054–1061. [Google Scholar] [CrossRef]
- Tsaban, G.; Yaskolka Meir, A.; Zelicha, H.; Rinott, E.; Kaplan, A.; Shalev, A.; Katz, A.; Brikner, D.; Blüher, M.; Ceglarek, U.; et al. Diet-Induced Fasting Ghrelin Elevation Reflects the Recovery of Insulin Sensitivity and Visceral Adiposity Regression. J. Clin. Endocrinol. Metab. 2022, 107, 336–345. [Google Scholar] [CrossRef]
- Zelicha, H.; Kaplan, A.; Yaskolka Meir, A.; Tsaban, G.; Rinott, E.; Shelef, I.; Tirosh, A.; Brikner, D.; Pupkin, E.; Qi, L.; et al. The Effect of Wolffia globosa Mankai, a Green Aquatic Plant, on Postprandial Glycemic Response: A Randomized Crossover Controlled Trial. Diabetes Care 2019, 42, 1162–1169. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of Green-Mediterranean Diet on Intrahepatic Fat: The DIRECT PLUS Randomised Controlled Trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of Phenolic Acid and Flavonoid Synthesis to Blue and Blue-Violet Light Depends on Plant Species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue Light Dosage Affects Carotenoids and Tocopherols in Microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]






| Compound(s) | 50 μmol m–2 s–1 | 1000 μmol m–2 s–1 | Sun-Exposed |
|---|---|---|---|
| Zeaxanthin | 0.02 ± 0.01 c | 0.48 ± 0.03 a | 0.34 ± 0.04 b |
| Lutein | 1.25 ± 0.05 a | 0.36 ± 0.00 c | 0.84 ± 0.03 b |
| β-carotene | 0.54 ± 0.06 a | 0.12 ± 0.01 c | 0.29 ± 0.03 b |
| α-tocopherol | 0.10 ± 0.02 a | 0.05 ± 0.01 b | 0.14 ± 0.02 a |
| Xanthophylls | 2.39 ± 0.07 a | 1.18 ± 0.01 c | 1.62 ± 0.07 b |
| Carotenoids | 2.92 ± 0.05 a | 1.31 ± 0.02 c | 1.91 ± 0.04 b |
| Xanthophylls/β-carotene | 4.58 ± 0.55 b | 9.61 ± 0.53 a | 5.57 ± 0.79 b |
| Zeaxanthin/Lutein | 0.01 ± 0.01 c | 1.35 ± 0.05 a | 0.40 ± 0.03 b |
| (V + A + Z)/Lutein | 0.59 ± 0.02 c | 2.15 ± 0.02 a | 0.64 ± 0.02 b |
| α-tocopherol/Carotenoids | 0.04 ± 0.01 b | 0.04 ± 0.01 b | 0.07 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polutchko, S.K.; Stewart, J.J.; McNamara, M.; Doherty Garcia, N.; López-Pozo, M.; Adams, W.W., III; Demmig-Adams, B. Lemna as a Sustainable, Highly Nutritious Crop: Nutrient Production in Different Light Environments. Nutraceuticals 2022, 2, 350-364. https://doi.org/10.3390/nutraceuticals2040027
Polutchko SK, Stewart JJ, McNamara M, Doherty Garcia N, López-Pozo M, Adams WW III, Demmig-Adams B. Lemna as a Sustainable, Highly Nutritious Crop: Nutrient Production in Different Light Environments. Nutraceuticals. 2022; 2(4):350-364. https://doi.org/10.3390/nutraceuticals2040027
Chicago/Turabian StylePolutchko, Stephanie K., Jared J. Stewart, Maureen McNamara, Naiara Doherty Garcia, Marina López-Pozo, William W. Adams, III, and Barbara Demmig-Adams. 2022. "Lemna as a Sustainable, Highly Nutritious Crop: Nutrient Production in Different Light Environments" Nutraceuticals 2, no. 4: 350-364. https://doi.org/10.3390/nutraceuticals2040027