Quantitative Assessment of Full Size Microplastics in Bottled and Tap Water Samples in Hong Kong
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Collection
2.2. Quality Control and Contamination Prevention
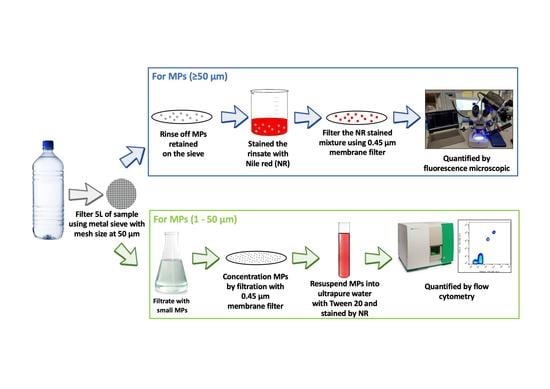
2.3. Pre-Concentration of MPs and Fluorescence Staining
2.4. Sample Quantification
2.5. Assessment of Potential MPs Contamination and Interference
2.6. Assessment of Potential Interence by Mineral Salts Addition
2.7. Data and Statistical Analysis
3. Results and Discussions
3.1. Abundance and Shapes of Large MPs (≥50 µm)
3.2. Abundance of Small MPs (1–50 µm)
3.3. Size Distribution of MPs in Bottled and Tap Water
3.4. Assessment of Potential MPs Contamination and Interference
3.5. Estimation of MPs Intake by Humans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, A.H.; Aris, A.Z.; Zaki, M.R.M.; Yusoff, F.M.; Wee, S.Y. Occurrence, potential sources and ecological risk estimation of microplastic towards coastal and estuarine zones in Malaysia. Mar. Pollut. Bull. 2022, 174, 113282. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Lusher, A.; Thompson, R.C.; Morley, A. The deposition and accumulation of microplastics in marine sediments and bottom water from the Irish continental shelf. Sci. Rep. 2017, 7, 10772. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Chen, X.; Zhang, K.; Mei, Z.; Hao, Y.; Zheng, J.; Wu, C.; Wang, K.; Ruan, Y.; Lam, P.K.; et al. Microplastics in the intestinal tracts of East Asian finless porpoises (Neophocaena asiaeorientalis sunameri) from Yellow Sea and Bohai Sea of China. Mar. Pollut. Bull. 2018, 136, 55–60. [Google Scholar] [CrossRef]
- Curren, E.; Leaw, C.P.; Lim, P.T.; Leong, S.C.Y. Evidence of marine microplastics in commercially harvested seafood. Front. Bioeng. Biotechnol. 2020, 8, 562760. [Google Scholar] [CrossRef]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; de Donno, A. Occurrence of microplastics in tap and bottled water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of microplastics on fish and in human health. Front. Environ. Sci. 2022, 10, 250. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Goodman, K.E.; Hare, J.T.; Khamis, Z.I.; Hua, T.; Sang, Q.X.A. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef]
- Bottled Water–China. Available online: https://www.statista.com/outlook/cmo/non-alcoholic-drinks/bottled-water/china (accessed on 11 May 2022).
- Zhou, X.J.; Wang, J.; Li, H.Y.; Zhang, H.M.; Zhang, D.L. Microplastic pollution of bottled water in China. J. Water Process Eng. 2021, 40, 101884. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic polymer contamination in bottled water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [Green Version]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Oliveri Conti, G. Exposure to microplastics (<10 µm) associated to plastic bottles mineral water consumption: The first quantitative study. Water Res. 2019, 157, 365–371. [Google Scholar] [CrossRef]
- Veerasingam, S.; Ranjani, M.; Venkatachalapathy, R.; Bagaev, A.; Mukhanov, V.; Litvinyuk, D.; Mugilarasan, M.; Gurumoorthi, K.; Guganathan, L.; Aboobacker, V.M.; et al. Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2681–2743. [Google Scholar] [CrossRef]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef] [Green Version]
- Tse, Y.-T.; Lo, H.-S.; Chan, S.M.-N.; Sze, E.T.-P. Flow cytometry as a rapid alternative to quantify small microplastics in environmental water samples. Water 2022, 14, 1436. [Google Scholar] [CrossRef]
- Kankanige, D.; Babel, S. Smaller-sized micro-plastics (MPs) contamination in single-use PET-bottled water in Thailand. Sci. Total Environ. 2020, 717, 137232. [Google Scholar] [CrossRef]
- Lam, T.W.L.; Ho, H.T.; Ma, A.T.; Fok, L. Microplastic Contamination of Surface Water-Sourced Tap Water in Hong Kong—A Preliminary Study. Appl. Sci. 2020, 10, 3463. [Google Scholar] [CrossRef]
- Feenstra, R.P.G. What is Actually Stained by Rose Bengal? Arch. Ophthalmol. 1992, 110, 984. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, K.; Cui, S.; Kang, Y.; An, L.; Lei, K. Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Res. 2019, 155, 175–181. [Google Scholar] [CrossRef]
- Wolff, S.; Weber, F.; Kerpen, J.; Winklhofer, M.; Engelhart, M.; Barkmann, L. Elimination of Microplastics by Downstream Sand Filters in Wastewater Treatment. Water 2020, 13, 33. [Google Scholar] [CrossRef]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane Processes for Microplastic Removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Weisser, J.; Beer, I.; Hufnagl, B.; Hofmann, T.; Lohninger, H.; Ivleva, N.P.; Glas, K. From the well to the bottle: Identifying sources of microplastics in mineral water. Water 2021, 13, 841. [Google Scholar] [CrossRef]
- Singh, T. Generation of microplastics from the opening and closing of disposable plastic water bottles. J. Water Health 2021, 19, 488–498. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A review of microplastics in table salt, drinking water, and air: Direct human exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef]
- Yaranal, N.A.; Subbiah, S.; Mohanty, K. Identification, extraction of microplastics from edible salts and its removal from contaminated seawater. Environ. Technol. Innov. 2021, 21, 101253. [Google Scholar] [CrossRef]
- Bottled Water–Hong Kong. Available online: https://www.statista.com/outlook/cmo/non-alcoholic-drinks/bottled-water/hong-kong#revenue (accessed on 14 September 2022).
- Report of the Second Hong Kong Population-Based Food Consumption Survey. Hong Kong Center of Food Safety. Available online: https://www.cfs.gov.hk/english/programme/programme_firm/files/2nd_FCS_Report_29_Jun_2021.pdf (accessed on 19 September 2022).
- Report of Population Health Survey 2014/2015. Hong Kong Center of Health Protection. Available online: https://www.chp.gov.hk/files/pdf/dh_phs_2014_15_full_report_eng.pdf (accessed on 19 September 2022).
- Samandra, S.; Mescall, O.J.; Plaisted, K.; Symons, B.; Xie, S.; Ellis, A.V.; Clarke, B.O. Assessing exposure of the Australian population to microplastics through bottled water consumption. Sci. Total Environ. 2022, 837, 155329. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]







| Sample | Brand | Origin | Water Type | Volume (L) | Material of Bottle |
|---|---|---|---|---|---|
| 1 | Best Buy | Hong Kong | Distilled water | 6 | PET |
| 2 | Watsons | Hong Kong | Distilled water | 4.5 | Recycle PET |
| 3 | Meadows | Malaysia | Distilled water | 1.5 | PET |
| 4 | SOC 99 | Japan | Spring water | 2 | PET |
| 5 | Highland Spring | Scotland | Spring water | 1.5 | PET |
| 6 | Meadows | France | Spring water | 1.5 | PET |
| 7 | Volvic | France | Mineral water | 1.5 | PET |
| 8 | Vittel | France | Mineral water | 1.5 | Recycle PET |
| 9 | Lotte | Korea | Mineral water | 2 | PET |
| Water Samples | 1–10 µm | ARA % | 10–50 µm | ARA % | ≥50 µm | ARA % |
|---|---|---|---|---|---|---|
| Best Buy | 1260 ± 883 | 80.5 ± 56.4 | 295 ± 280 | 18.8 ± 17.9 | 11 ± 2 | 0.7 ± 0.1 |
| Watsons | 4667 ± 1620 | 98.4 ± 34.1 | 48 ± 114 | 1.0 ± 2.4 | 29 ± 19 | 0.6 ± 0.4 |
| Meadows (D) | 10,640 ± 3187 | 98.2 ± 29.4 | 188 ± 42 | 1.7 ± 0.4 | 11 ± 3 | 0.1 ± 0.03 |
| SOC 99 | 9707 ± 2290 | 97.8 ± 23.1 | 202 ± 170 | 2.0 ± 1.7 | 12 ± 2 | 0.1 ± 0.02 |
| Highland Spring | 2660 ± 2393 | 93.5 ± 84.1 | 175 ± 92 | 6.2 ± 3.2 | 9 ± 5 | 0.3 ± 0.2 |
| Meadows (S) | 7600 ± 2754 | 91.6 ± 33.2 | 668 ± 304 | 8.1 ± 3.7 | 27 ± 5 | 0.3 ± 0.1 |
| Volvic | 11,060 ± 5669 | 95.4 ± 48.9 | 488 ± 201 | 4.2 ± 1.7 | 50 ± 21 | 0.4 ± 0.2 |
| Lotte | 17,393 ± 4304 | 97.2 ± 24.1 | 488 ± 232 | 2.7 ± 1.3 | 8 ± 4 | 0.04 ± 0.02 |
| Vittel | 12,787 ± 7988 | 97.3 ± 60.8 | 315 ± 251 | 2.4 ± 1.9 | 39 ± 11 | 0.3 ± 0.1 |
| Tap water A | 1533 ± 1094 | 82.8 ± 59.1 | 300 ± 100 | 16.2 ± 5.4 | 18 ± 11 | 1.0 ± 0.6 |
| Tap water B | 1693 ± 488 | 97.2 ± 28.0 | 40 ± 40 | 2.3 ± 2.3 | 9 ± 3 | 0.5 ± 0.2 |
| Tap water C | 927 ± 497 | 53.4 ± 28.6 | 800 ± 20 | 46.1 ± 1.2 | 9 ± 5 | 0.5 ± 0.3 |
| Average | 6827 ± 5483 | 95.1 ± 76.4 | 334 ± 236 | 4.7 ± 3.3 | 19 ± 14 | 0.3 ± 0.2 |
| Hong Kong (Bottled Water) | Hong Kong (Tap Water) | Hong Kong (Total) | |
|---|---|---|---|
| IR (L day−1) | 0.310 | 0.869 | 1.179 |
| bw (kg) | 62.3 | ||
| Average concentration of ≥50 µm MPs (MPs L−1) | 22 | 12 | - |
| Average concentration of ≥25 µm MPs (MPs L−1) | 34 | 28 | - |
| Average concentration of <50 µm MPs (MPs L−1) | 8934 | 1753 | - |
| Calculated EDI of ≥50 µm MPs | 0.109 | 0.167 | 0.276 |
| Calculated EDI of <50 µm MPs | 44.455 | 24.463 | 68.918 |
| Calculated EDI of 1 µm to 5 mm MPs | 44.564 | 24.630 | 69.194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, Y.-T.; Chan, S.M.-N.; Sze, E.T.-P. Quantitative Assessment of Full Size Microplastics in Bottled and Tap Water Samples in Hong Kong. Int. J. Environ. Res. Public Health 2022, 19, 13432. https://doi.org/10.3390/ijerph192013432
Tse Y-T, Chan SM-N, Sze ET-P. Quantitative Assessment of Full Size Microplastics in Bottled and Tap Water Samples in Hong Kong. International Journal of Environmental Research and Public Health. 2022; 19(20):13432. https://doi.org/10.3390/ijerph192013432
Chicago/Turabian StyleTse, Yuet-Tung, Sidney Man-Ngai Chan, and Eric Tung-Po Sze. 2022. "Quantitative Assessment of Full Size Microplastics in Bottled and Tap Water Samples in Hong Kong" International Journal of Environmental Research and Public Health 19, no. 20: 13432. https://doi.org/10.3390/ijerph192013432