Effects of Agricultural Management of Spent Mushroom Waste on Phytotoxicity and Microbiological Transformations of C, P, and S in Soil and Their Consequences for the Greenhouse Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Experimental Setup and Soil Sampling
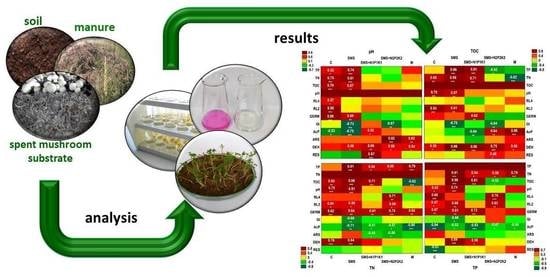
- Soil without fertilizer (control object) (C);
- Soil + spent mushroom substrate (SMS);
- Soil + spent mushroom substrate + N1P1K1 (SMS + N1P1K1);
- Soil + spent mushroom substrate + N2P2K2 (SMS + N2P2K2);
- Soil + cattle manure (M).
2.2. Meteorological Conditions
2.3. Biochemical and Enzymatic Analyses
2.4. Phytotoxicity
2.5. Chemical Analyses
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adejumo, I.O.; Adebiyi, O.A. Agricultural solid wastes: Causes, effects, and effective management. In Strategies of Sustainable Solid Waste Management; Saleh, H.M., Ed.; Intech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Hanafi, M.F.H.; Rezania, S.; Taib, M.S.; Md Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. 2022. Available online: https://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 28 July 2022).
- Cunha Zied, D.; Sánchez, J.E.; Noble, R.; Pardo-Giménez, A. Use of spent mushroom substrate in new mushroom crops to promote the transition towards a circular economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef] [PubMed]
- Rinker, D.L. Spent mushroom substrate uses. In Edible and Medicinal Mushrooms: Technology and Applications; Cunha Zied, D., Pardo-Gimenez, A., Eds.; Eds.; Wiley-Blackwell: West Sussex, UK, 2017; pp. 427–454. [Google Scholar]
- Kwiatkowski, C.A.; Harasim, E. The Effect of Fertilization with Spent Mushroom Substrate and Traditional Methods of Fertilization of Common Thyme (Thymus vulgaris L.) on Yield Quality and Antioxidant Properties of Herbal Material. Agronomy 2021, 11, 329. [Google Scholar] [CrossRef]
- Owaid, M.N.; Abed, I.A.; Al-Saeedi, S.S.S. Applicable properties of the bio-fertilizer spent mushroom substrate in organic systems as a byproduct from the cultivation of Pleurotus spp. Inform. Process. Agric. 2017, 4, 78–82. [Google Scholar] [CrossRef]
- Prasad, R.; Lisiecka, J.; Kleiber, T. Morphological and Yield Parameters, Dry Matter Distribution, Nutrients Uptake, and Distribution in Strawberry (Fragaria × ananassa Duch.) cv. ‘Elsanta’ as Influenced by Spent Mushroom Substrates and Planting Seasons. Agronomy 2022, 12, 854. [Google Scholar] [CrossRef]
- Velusami, B.; Jordan, S.N.; Curran, T.; Grogan, H. Fertiliser characteristics of stored spent mushroom substrate as a sustainable source of nutrients and organic matter for tillage, grassland and agricultural soils. Irish, J. Agric. Food Res. 2021, 60, 1–11. [Google Scholar] [CrossRef]
- Frąc, M.; Pertile, G.; Panek, J.; Gryta, A.; Oszust, K.; Lipiec, J.; Usowicz, B. Mycobiome composition and diversity under the long-term application of spent mushroom substrate and chicken manure. Agronomy 2021, 11, 410. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, H.; Zhang, Q.; Wang, Q.; Chen, C.; Mooney, S.J.; Peng, X.; Du, Z. Biochar enhances soil hydraulic function but not soil aggregation in a sandy loam. Eur. J. Soil Sci. 2019, 70, 291–300. [Google Scholar] [CrossRef]
- Lipiec, J.; Usowicz, B.; Kłopotek, J.; Turski, M.; Frąc, M. Effects of Application of Recycled Chicken Manure and Spent Mushroom Substrate on Organic Matter, Acidity, and Hydraulic Properties of Sandy Soils. Materials 2021, 14, 4036. [Google Scholar] [CrossRef]
- Malińska, K.; Czekała, W.; Janczak, D.; Dach, J.; Mazurkiewicz, J.; Dróżdż, D. Spent mushroom substrate as a supplementary material for sewage sludge composting mixtures. Environ. Prot. Eng. 2018, 21, 29–38. [Google Scholar] [CrossRef]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Zhang, S.; Gong, H.; Zhang, X.; Wu, C.; Li, W. Improving sewage sludge composting by addition of spent mushroom substrate and sucrose. Bioresour. Technol. 2018, 253, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kucaj, W.F.; Rygielski, K.; Cybulska, K. Optimizing the use of the phytotoxkit test to assess the toxicity of soil contaminated with creosote. Pol. J. Soil Sci. 2019, 52, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Szymanski, M.; Dobrucka, R. Application of Phytotests to Study of Environmental Safety of Biologically Synthetised Au and Au/ZnO Nanoparticles Using Tanacetum parthenium Extract. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1354–1369. [Google Scholar] [CrossRef]
- Alvarenga, P.; Mourinha, C.; Farto, M.; Santos, T.; Palma, P.; Sengo, J.; Morais, M.-C.; Cunda-Queda, C. Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: Benefits versus limiting factors. Waste Manag. 2015, 40, 44–52. [Google Scholar] [CrossRef]
- Godlewska, P.; Jośko, I.; Oleszczuk, P. Ecotoxicity of sewage sludge- or sewage sludge/willow-derived biochar-amended soil. Environ. Pollut. 2022, 305, 119235. [Google Scholar] [CrossRef]
- Joniec, J.; Oleszczuk, P.; Jezierska-Tys, S.; Kwiatkowska, E. Effect of reclamation treatments on microbial activity and phytotoxicity of soil degraded by the sulphur mining industry. Environ Pollut. 2019, 252, 1429–1438. [Google Scholar] [CrossRef]
- Manas, P.; De las Heras, J. Phytotoxicity test applied to sewage sludge using Lactuca sativa L. and Lepidium sativum L. seeds. Int. J. Environ. Sci. Technol. 2018, 15, 273–280. [Google Scholar] [CrossRef]
- Pampuro, N.; Bisaglia, C.; Romano, E.; Brambilla, M.; Foppa Pedretti, E.; Cavallo, E. Phytotoxicity and Chemical Characterization of Compost Derived from Pig Slurry Solid Fraction for Organic Pellet Production. Agriculture 2017, 7, 94. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.; Pascual, K.S.; Romasanta, R.R.; Van Trinh, M.; Van Thach, T.; Van Hung, N.; Chivenge, P. Rice straw management effects on greenhouse gas emissions and mitigation options. In Sustainable Rice Straw Management; Gummert, M., Hung, N., Chivenge, P., Douthwaite, B., Eds.; Springer: Cham, Switzerland, 2020; pp. 145–159. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M. Carbon dioxide emission from soil. Agric. Res. 2013, 2, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Navarro-Pedreño, J.; Almendro-Candel, M.B.; Zorpas, A.A. The Increase of Soil Organic Matter Reduces Global Warming, Myth or Reality? Science 2021, 3, 18. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global carbon budget. Earth Syst. Sci. Data 2013, 6, 235–263. [Google Scholar] [CrossRef] [Green Version]
- Joshi Gyawali, A.; Lester, B.J.; Stewart, R.D. Talking SMAAC: A new tool to measure soil respiration and microbial activity. Front. Earth Sci. 2019, 7, 138. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Hilton, S.L.; Bending, G.D.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in activity and structure of the soil microbial community after application of azoxystrobin or pirimicarb and an organic amendment to an agricultural soil. App. Soil Ecol. 2016, 106, 47–57. [Google Scholar] [CrossRef]
- Elsakhawy, T.A.; El-Rahem, W.T.A. Evaluation of Spent Mushroom Substrate Extract as a Biofertilizer for Growth Improvement of Rice (Oryza sativa L). Egypt. J. Soil Sci. 2020, 60, 31–42. [Google Scholar] [CrossRef]
- Joniec, J.; Żukowska, G.; Bik-Małodzińska, M.; Kwiatkowska, E.; Rojek, K. Reaction of Microorganisms to Long-Term Waste Reclamation of Soil Degraded by the Sulfur Mining Industry. Minerals 2021, 11, 1226. [Google Scholar] [CrossRef]
- Paula, F.S.; Tatti, E.; Abram, F.; Wilson, J.; O’Flaherty, V. Stabilisation of spent mushroom substrate for application as a plant growth-promoting organic amendment. J. Environ. Manag. 2017, 196, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Mooshammer, M.; Sauvadet, M.; Meng, C.; Alvarez, G.; Bernard, L.; Bertrand, I.; Blagodatskaya, E.; Bon, L.; Fontaine, S.; et al. Soil enzymes in response to climate warming: Mechanisms and feedbacks. Funct. Ecol. 2022, 36, 1378–1395. [Google Scholar] [CrossRef]
- Zi, H.B.; Hu, L.; Wang, C.; Wang, G.; Wu, P.; Lerdau, M.; Ade, L. Responses of soil bacterial community and enzyme activity to experimental warming of an alpine meadow. Eur. J. Soil Sci. 2018, 69, 429–438. [Google Scholar] [CrossRef]
- Meng, C.; Tian, D.S.; Zeng, H.; Li, Z.L.; Chen, H.Y.H.; Niu, S.L. Global meta-analysis on the responses of soil extracellular enzyme activities to Warming. Sci. Total Environ. 2020, 705, 135992. [Google Scholar] [CrossRef]
- Shi, L.; Guo, Y.; Ning, J.; Lou, S.; Hou, F. Herbicide applications increase greenhouse gas emissions of alfalfa pasture in the inland arid region of northwest China. PeerJ 2020, 8, e9231. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Jiang, L.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Zhang, H.; Tan, W.W.; Gao, J.L.; Hou, A.X. Microbial abundance and enzymatic activity from tussock and shrub soil in permafrost peatland after 6-year warming. Ecol. Indic. 2021, 126, 107589. [Google Scholar] [CrossRef]
- Alvarenga, P.; Rodrigues, D.; Mourinha, C.; Palma, P.; de Varennes, A.; Cruz, N.; Tarelho, L.A.C.; Rodrigues, S. Use of wastes from the pulp and paper industry for the remediation of soils degraded by mining activities: Chemical, biochemical and ecotoxicological effects. Sci. Total Environ. 2019, 686, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Cheviron, N.; Amadou, I.; Grondin, V.; Marrauld, C.; Mougin, C.; Morvan, T. Soil enzymatic activity data over eight years at the EFELE site, a long-term field experiment on effects of organic waste products and tillage practices. Data Brief. 2021, 36, 106959. [Google Scholar] [CrossRef]
- Joniec, J. Enzymatic activity as an indicator of regeneration processes in degraded soil reclaimed with various types of waste. Int. J. Environ. Sci. Technol. 2018, 15, 2241–2252. [Google Scholar] [CrossRef] [Green Version]
- Joniec, J.; Kwiatkowska, E.; Kwiatkowski, C.A. Assessment of the Effects of Soil Fertilization with Spent Mushroom Substrate in the Context of Microbial Nitrogen Transformations and the Potential Risk of Exacerbating the Greenhouse Effect. Agriculture 2022, 12, 1190. [Google Scholar] [CrossRef]
- Lal, R.; Bouma, J.; Brevik, E.; Dawson, L.; Field, D.J.; Glaser, B.; Hatano, R.; Hartemink, A.E.; Kosaki, T.; Lascelles, B.; et al. Soils and Sustainable Development Goals of the United Nations: An International Union of Soil Sciences Perspective. Geoderma Reg. 2021, 25, e00398. [Google Scholar] [CrossRef]
- Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 14 September 2022).
- Polish Society of Soil Science. Particle size distribution and textural classes of soils and mineral materials—Classification of Polish Society of Soil Science 2008. Soil Sci. Ann. 2009, 60, 5–16. [Google Scholar]
- WRB IUSS Working Group. World Reference Base for Soil Resources 2014. update 2015; International soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Reports; FAO: Rome, Italy, 2015. [Google Scholar]
- Rühling, A.; Tyler, G. Heavy metal pollutions and decomposition of Spruce Needle litter. Oikos 1973, 24, 402–415. [Google Scholar] [CrossRef]
- Thalmann, A. Zur Methodik der Bestimmung der dehydrogenaseactivität im boden mittels triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylosulfatase activity of soils. Soil Sci. Soc. Am. J. 1970, 34, 225–229. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Garcia, C. Soil agro-ecological management: Fertirrigation and vermicompost treatments. Bioresour. Technol. 1997, 59, 199–206. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental Factors Affecting the Mineralization of Crop Residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Dai, S.; Hower, J.C.; Finkelman, R.B.; Graham, I.T.; French, D.; Ward, C.R.; Eskenzay, G.; Wei, Q.; Zhao, L. Organic associations of non-mineral elements in coal: A review. Int. J. Coal. Geol. 2020, 218, 103347. [Google Scholar] [CrossRef]
- Hernandez, T.; Berlanga, J.G.; Tormos, I.; Garcia, C. Organic versus Inorganic Fertilizers: Response of Soil Properties and Crop Yield. AIMS Geosci. 2021, 7, 415–439. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173–174, 152–161. [Google Scholar] [CrossRef]
- Chen, J.; Song, D.; Liu, D.; Sun, J.; Wang, X.; Zhou, W.; Liang, G. Soil Aggregation Shaped the Distribution and Interaction of Bacterial-Fungal Community Based on a 38-Year Fertilization Experiment in China. Front Microbiol. 2022, 13, 824681. [Google Scholar] [CrossRef] [PubMed]
- Kátai, J.; Zsuposné, A.O.; Tállai, M.; Alshaal, T. Would fertilization history render the soil microbial communities and their activities more resistant to rainfall fluctuations? Ecotoxicol. Environ. Saf. 2020, 201, 110803. [Google Scholar] [CrossRef] [PubMed]
- Sivojiene, D.; Kacergius, A.; Baksiene, E.; Maseviciene, A.; Zickiene, L. The influence of organic fertilizers on the abundance of soil microorganism communities, agrochemical indicators, and yield in east lithuanian light soils. Plants 2021, 10, 2648. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Chorover, J. Solute release from weathering of spent mushroom substrate under controlled conditions. Compost. Sci. Util. 2004, 12, 225–234. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A. Soil Enzyme Activity Response under the Amendment of Different Types of Biochar. Agronomy 2022, 12, 569. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M.A. Enzymes in Agricultural Sciences; OMICS International: Hyderabad, India, 2014. [Google Scholar]
- Hu, H.; Zhou, H.; Zhou, S.; Li, Z.; Wei, C.; Yu, Y.; Hay, A.G. Fomesafen impacts bacterial communities and enzyme activities in the rhizosphere. Environ. Pollut. 2019, 253, 302–311. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases; Canuto, R.A., Ed.; InTech: Singapore, 2012; Volume 10, pp. 183–210. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Li, S.; Carson, M.A.; Chang, S.X.; Wu, Q.; Wang, L.; An, Z.; Sun, X. Spent mushroom substrate and cattle manure amendments enhance the transformation of garden waste into vermicomposts using the earthworm Eisenia fetida. J. Environ. Manag. 2019, 248, 109263. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Cano, A.; Johnson, J. Simultaneous determination of multiple soil enzyme activities for soil health-biogeochemical indices. Appl. Soil Ecol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of soil enzymes in sustainable crop production. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Kudus, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2019; pp. 569–589. [Google Scholar] [CrossRef]
- Manzoor, A.; Dippold, M.A.; Loeppmann, S.; Blagodatskaya, E. Two-Phase Conceptual Framework of Phosphatase Activity and Phosphorus Bioavailability. Front. Plant. Sci. 2022, 13, 935829. [Google Scholar] [CrossRef]
- Perez-de-Mora, A.; Burgos, P.; Cabrera, F.; Madejon, E. Progress in microbial activity and chemical properties of a trace element polluted soil dunder assisted natural remediation. In Soil Enzymology in the Recycling of Organic Wastes and Environmental Restoration; Trasar-Cepeda, C., Hernandez, T., Garcia, C., Gonzalez-Carcedo, S., Eds.; Springer: Berlin, Germany, 2012; pp. 167–179. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Zhou, K.; Wang, C.; Wang, S.; Wang, Y.; Zheng, M.; Lu, X.; Zhang, W.; Mo, J. Effects of 14-year continuous nitrogen addition on soil arylsulphatase and phosphodiesterase activities in a mature tropical forest. Glob. Ecol. Conserv. 2020, 22, e00934. [Google Scholar] [CrossRef]
- Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- Catal, S.; Peksen, A. Physical, chemical and biological properties of spent mushroom substrates of different mushroom species. Acta Hortic. 2020, 1287, 353–360. [Google Scholar] [CrossRef]
- Mohamed, E.A.A.; Muddathir, A.M.; Abdalla, A.H. Effects of organic and inorganic fertilization on growth, yield, seed fixed oil content, and fatty acids profile of garden cress (Lepidium sativum L.). SN Appl. Sci. 2020, 2, 1753. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as a plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 1–11. [Google Scholar] [CrossRef]













| Property | Unit | Soil | Spent Mushroom Substrate | Manure |
|---|---|---|---|---|
| pHKCl | 1 mol KCl | 7.0 | 6.6 | 7.3 |
| TOC | g kg−1 | 14.98 | 105.0 | 135.8 |
| TN | g kg−1 | 1.51 | 6.50 | 9.47 |
| TP | g kg−1 | 0.19 | 0.25 | 0.25 |
| Ca | mg kg−1 | 1660 | 15,800 | 2240 |
| K | 2350 | 6330 | 11,100 | |
| Mg | 1390 | 1240 | 1550 | |
| Zn | mg kg−1 | No. | 86.0 | No. |
| Cu | 16.6 | |||
| Ni | 2.81 | |||
| Cr | 1.84 | |||
| Cd | 0.055 | |||
| Pb | 0.956 | |||
| Hg | 0.07 |
| Year | Season | C | SMS | SMS + N1P1K1 | SMS + N2P2K2 | M | |
|---|---|---|---|---|---|---|---|
| pH 1 mol KCl | 2018 | spring | 7.03 | 7.20 | 6.41 | 5.16 | 7.47 |
| autumn | 6.86 | 7.60 | 5.98 | 6.60 | 5.44 | ||
| 2019 | spring | 6.42 | 6.75 | 5.88 | 5.84 | 6.20 | |
| autumn | 6.34 | 6.04 | 6.18 | 5.53 | 6.24 | ||
| 2020 | spring | 6.87 | 6.85 | 6.68 | 6.79 | 6.56 | |
| autumn | 6.25 | 6.13 | 6.33 | 6.64 | 6.50 | ||
| TOC g kg−1 | 2018 | spring | 14.98 | 19.50 | 17.21 | 12.83 | 13.45 |
| autumn | 13.59 | 14.39 | 14.34 | 11.46 | 12.16 | ||
| 2019 | spring | 12.19 | 12.99 | 14.75 | 15.60 | 14.89 | |
| autumn | 12.02 | 10.63 | 13.25 | 13.28 | 18.18 | ||
| 2020 | spring | 15.62 | 16.30 | 14.90 | 15.33 | 17.75 | |
| autumn | 13.34 | 12.54 | 13.85 | 14.91 | 14.78 | ||
| TN g kg−1 | 2018 | spring | 1.51 | 1.82 | 2.13 | 1.46 | 1.36 |
| autumn | 1.37 | 1.44 | 1.39 | 1.18 | 1.28 | ||
| 2019 | spring | 1.50 | 1.10 | 1.00 | 1.30 | 1.10 | |
| autumn | 0.96 | 0.97 | 1.30 | 0.84 | 1.00 | ||
| 2020 | spring | 1.70 | 1.20 | 0.98 | 1.40 | 1.10 | |
| autumn | 0.97 | 0.80 | 1.20 | 0.55 | 1.10 | ||
| TP g kg−1 | 2018 | spring | 0.19 | 0.21 | 0.21 | 0.17 | 0.22 |
| autumn | 0.16 | 0.16 | 0.14 | 0.15 | 0.18 | ||
| 2019 | spring | 0.15 | 0.13 | 0.19 | 0.10 | 0.10 | |
| autumn | 0.11 | 0.10 | 0.11 | 0.13 | 0.15 | ||
| 2020 | spring | 0.10 | 0.15 | 0.12 | 0.16 | 0.15 | |
| autumn | 0.12 | 0.13 | 0.14 | 0.11 | 0.14 |
| Years | Experimental Treatments | RES | DEH | AcP | ARS | GI | GERM | RL2 | RL4 |
|---|---|---|---|---|---|---|---|---|---|
| 2018 | C | 67.28 a | 4.89 abc | 36.60 b | 63.95 i | 100.00 ab | 99.33 g | 1.99 f | 3.74 g |
| SMS | 80.58 cd | 11.60 i | 38.58 b | 60.43 h | 92.86 a | 99.00 g | 1.70 de | 2.92 de | |
| SMS + N1P1K1 | 143.84 h | 9.23 gh | 34.25 b | 27.44 c | 93.75 a | 99.17 g | 1.62 de | 2.83 cde | |
| SMS + N2P2K2 | 111.40 f | 3.77 a | 24.35 a | 23.58 a | 176.86 e | 99.00 g | 1.57 d | 2.98 def | |
| M | 89.65 d | 4.88 ab | 23.60 a | 31.28 d | 145.86 d | 98.50 g | 1.50 cd | 1.99 ab | |
| 2019 | C | 80.75 cd | 5.31 bc | 48.76 c | 42.58 ef | 100.00 ab | 88.00 de | 1.18 ab | 3.13 efg |
| SMS | 77.76 bc | 3.94 a | 48.93 c | 45.51 g | 220.27 f | 88.42 ef | 1.30 bc | 2.50 bcd | |
| SMS + N1P1K1 | 101.64 e | 4.64 ab | 43.92 c | 26.50 bc | 144.20 d | 87.00 cde | 1.15 ab | 2.09 ab | |
| SMS + N2P2K2 | 100.92 e | 4.37 ab | 57.06 de | 24.65 ab | 116.94 abc | 90.58 f | 1.00 a | 1.65 a | |
| M | 70.38 ab | 6.01 cd | 55.59 d | 40.23 e | 181.52 e | 88.83 ef | 1.30 bc | 2.24 abc | |
| 2020 | C | 121.85 g | 6.72 de | 61.98 ef | 46.72 g | 100.00 ab | 85.00 bc | 1.59 d | 2.55 bcde |
| SMS | 121.63 g | 7.80 ef | 62.14 ef | 42.06 ef | 134.31 cd | 83.50 b | 1.52 cd | 2.55 bcde | |
| SMS + N1P1K1 | 234.88 i | 9.66 h | 67.60 g | 41.06 e | 145.80 d | 85.50 bc | 1.87 ef | 3.55 fg | |
| SMS + N2P2K2 | 237.80 i | 8.77 fgh | 65.00 fg | 44.56 fg | 92.83 a | 85.75 bcd | 1.84 ef | 3.03 def | |
| M | 116.22 fg | 8.25 fg | 64.69 fg | 46.56 g | 122.13 bcd | 80.25 a | 1.99 f | 3.04 def |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, E.; Joniec, J. Effects of Agricultural Management of Spent Mushroom Waste on Phytotoxicity and Microbiological Transformations of C, P, and S in Soil and Their Consequences for the Greenhouse Effect. Int. J. Environ. Res. Public Health 2022, 19, 12915. https://doi.org/10.3390/ijerph191912915
Kwiatkowska E, Joniec J. Effects of Agricultural Management of Spent Mushroom Waste on Phytotoxicity and Microbiological Transformations of C, P, and S in Soil and Their Consequences for the Greenhouse Effect. International Journal of Environmental Research and Public Health. 2022; 19(19):12915. https://doi.org/10.3390/ijerph191912915
Chicago/Turabian StyleKwiatkowska, Edyta, and Jolanta Joniec. 2022. "Effects of Agricultural Management of Spent Mushroom Waste on Phytotoxicity and Microbiological Transformations of C, P, and S in Soil and Their Consequences for the Greenhouse Effect" International Journal of Environmental Research and Public Health 19, no. 19: 12915. https://doi.org/10.3390/ijerph191912915