Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Ethical Statements
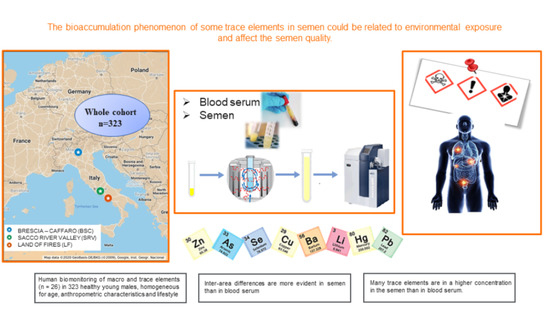
2.3. General Description
2.4. Semen and Serum Collection
2.5. Macro and Trace Elements Analysis
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSC | Brescia-Caffaro area |
| SRV | Sacco River Valley area |
| LF | Land of Fires area |
| WRC | Whole Recruited Cohort |
| ATSDR | Agency for Toxic Substances and Disease Registry |
| IARC | International Agency for Research on Cancer |
| WHO | World Health Organization |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| SIVR | Italian Society of Reference Values |
| SIN | Sites of National Interest |
| ISS | Italian National Institute of Health |
| ROS | Reactive Oxygen Species |
| CRM | Certified Reference Materials |
| IRMM | Institute for Reference Materials and Measurements |
References
- Frieden, E. New Perspectives on the Essential Trace Elements. J. Chem. Educ. 1985, 62, 917. [Google Scholar] [CrossRef]
- Frieden, E. The Chemical Elements of Life. Sci. Am. 1972, 227, 52–60. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations; International Atomic Energy Agency (Eds.) Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996; ISBN 978-92-4-156173-0. [Google Scholar]
- Prashanth, L.; Kattapagari, K.K.; Chitturi, R.T.; Baddam, V.R.R.; Prasad, L.K. A Review on Role of Essential Trace Elements in Health and Disease. J. Dr. NTR Univ. Health Sci. 2015, 4, 75. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Fraga, C.G. Relevance, Essentiality and Toxicity of Trace Elements in Human Health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Karalliedde, L.; Brooke, N. Toxicity of Heavy Metals and Trace Elements. In Essentials of Toxicology for Health Protection; Baker, D., Karalliedde, L., Murray, V., Maynard, R., Parkinson, N.H., Eds.; Oxford University Press: Oxford, UK, 2012; ISBN 978-0-19-965254-9. [Google Scholar]
- ATSDR Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 9 September 2020).
- Virgolini, M.B.; Aschner, M. Chapter Five—Molecular Mechanisms of Lead Neurotoxicity. In Advances in Neurotoxicology; Aschner, M., Costa, L.G., Eds.; Neurotoxicity of Metals: Old Issues and New Developments; Academic Press: Cambridge, MA, USA, 2021; Volume 5, pp. 159–213. [Google Scholar]
- Smith, C.J.; Livingston, S.D.; Doolittle, D.J. An International Literature Survey of “IARC Group I Carcinogens” Reported in Mainstream Cigarette Smoke. Food Chem. Toxicol. 1997, 35, 1107–1130. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Impact of Environmental Factors on Human Semen Quality and Male Fertility: A Narrative Review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [Green Version]
- Mann, U.; Shiff, B.; Patel, P. Reasons for Worldwide Decline in Male Fertility. Curr. Opin. Urol. 2020, 30, 296–301. [Google Scholar] [CrossRef]
- Bergamo, P.; Volpe, M.G.; Lorenzetti, S.; Mantovani, A.; Notari, T.; Cocca, E.; Cerullo, S.; Di Stasio, M.; Cerino, P.; Montano, L. Human Semen as an Early, Sensitive Biomarker of Highly Polluted Living Environment in Healthy Men: A Pilot Biomonitoring Study on Trace Elements in Blood and Semen and Their Relationship with Sperm Quality and RedOx Status. Reprod. Toxicol. 2016, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kasperczyk, A.; Dobrakowski, M.; Horak, S.; Zalejska-Fiolka, J.; Birkner, E. The Influence of Macro and Trace Elements on Sperm Quality. J. Trace Elem. Med. Biol. 2015, 30, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires. A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Trifuoggi, M.; Scarano, C.; Gori, C.; Giarra, A.; Febbraio, F. Relevance of Arginine Residues in Cu(II)-Induced DNA Breakage and Proteinase K Resistance of H1 Histones. Sci. Rep. 2018, 8, 7414. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Curry, B.J. Redox Regulation of Human Sperm Function: From the Physiological Control of Sperm Capacitation to the Etiology of Infertility and DNA Damage in the Germ Line. Antioxid. Redox Signal. 2010, 14, 367–381. [Google Scholar] [CrossRef]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive Oxygen Species and Sperm Function—In Sickness and In Health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Montano, L.; Bergamo, P.; Lorenzetti, S.; Andreassi, M.G. The Role of Human Semen as an Early and Reliable Tool of Environmental Impact Assessment on Human Health. In Spermatozoa—Facts Perspect; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Montano, L. Reproductive Biomarkers as Early Indicators for Assessing Environmental Health Risk. Available online: https://www.eurekaselect.com/185279/chapter (accessed on 20 December 2020).
- Effects of Lifestyle Changes on Semen Quality in Healthy Young Men Living in Highly Polluted Areas (FAST)—Full Text View. ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04012385 (accessed on 11 September 2020).
- Montano, L.; Ceretti, E.; Donato, F.; Bergamo, P.; Zani, C.; Viola, G.C.V.; Notari, T.; Pappalardo, S.; Zani, D.; Ubaldi, S.; et al. Effects of a Lifestyle Change Intervention on Semen Quality in Healthy Young Men Living in Highly Polluted Areas in Italy: The FASt Randomized Controlled Trial. Eur. Urol. Focus 2021, 8, 351–359. [Google Scholar] [CrossRef]
- WMA—The World Medical Association. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Jahrb. Für Wiss. Und Ethik 2009, 14, 233–238. [Google Scholar] [CrossRef]
- Ecosistema Urbano 2018, il Rapporto di Legambiente Sulle Performance Ambientali Delle Città Capoluogo; Legambiente: Roma, Italy, 2018.
- Di Guardo, A.; Terzaghi, E.; Raspa, G.; Borin, S.; Mapelli, F.; Chouaia, B.; Zanardini, E.; Morosini, C.; Colombo, A.; Fattore, E.; et al. Differentiating Current and Past PCB and PCDD/F Sources: The Role of a Large Contaminated Soil Site in an Industrialized City Area. Environ. Pollut. 2017, 223, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; Magoni, M.; Bergonzi, R.; Scarcella, C.; Indelicato, A.; Carasi, S.; Apostoli, P. Exposure to Polychlorinated Biphenyls in Residents near a Chemical Factory in Italy: The Food Chain as Main Source of Contamination. Chemosphere 2006, 64, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Fantini, F.; Porta, D.; Fano, V.; De Felip, E.; Senofonte, O.; Abballe, A.; D’Ilio, S.; Ingelido, A.M.; Mataloni, F.; Narduzzi, S.; et al. Epidemiologic studies on the health status of the population living in the Sacco River Valley. Epidemiol. Prev. 2012, 36, 44–52. [Google Scholar]
- Cembalo, L.; Caso, D.; Carfora, V.; Caracciolo, F.; Lombardi, A.; Cicia, G. The “Land of Fires” Toxic Waste Scandal and Its Effect on Consumer Food Choices. Int. J. Environ. Res. Public Health 2019, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Senior, K.; Mazza, A. Italian “Triangle of Death” Linked to Waste Crisis. Lancet Oncol. 2004, 5, 525–527. [Google Scholar] [CrossRef]
- Frieden, E. The Evolution of Metals as Essential Elements [with Special Reference to Iron and Copper]. In Protein-Metal Interactions; Friedman, M., Ed.; Advances in Experimental Medicine and Biology; Springer New York: Boston, MA, USA, 1974; pp. 1–31. ISBN 978-1-4684-0943-7. [Google Scholar]
- Adoamnei, E.; Mendiola, J.; Moñino-García, M.; López-Espín, J.J.; Navarrete-Muñoz, E.M.; Torres-Cantero, A.M. Oligoelementos en la dieta y calidad seminal y niveles de hormonas reproductivas en varones jóvenes: Relación con la fertilidad. Rev. Int. Androl. 2019, 17, 46–54. [Google Scholar] [CrossRef]
- Alimonti, A.; Bocca, B.; Ruggieri, F. Reference Method for the Determination of Chemical Elements in Human Biological Matrices: Analytical Performances and Uncertainty of Data; (Rapporti ISTISAN 15/30); Istituto Superiore Di Sanità: Roma, Italy, 2015. [Google Scholar]
- Perrone, P.; Longo, V.; Capone, S.; Forleo, A.; Pappalardo, S.; Montano, L.; Piscopo, M. Molecular Alteration and Severe Abnormalities in Spermatozoa of Young Men Living in the Valley of Sacco River (Latium, Italy): A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 11023. [Google Scholar] [CrossRef]
- SIVR—Società Italiana Dei Valori Di Riferimento. Available online: http://www.sivr.it/documenti.htm (accessed on 20 December 2020).
- Alimonti, A.; Bocca, B.; Mattei, D.; Pino, A. Programma per Il Biomonitoraggio Dell’esposizione Della Popolazione Italiana (PROBE): Dose Interna Dei Metalli. Roma:Istituto Superiore Di Sanità (Rapporti ISTISAN 11/9 IT). Available online: http://old.iss.it/binary/publ/cont/11_9IT_web.pdf (accessed on 15 September 2020).
- Alimonti, A.; Bocca, B.; Mattei, D.; Pino, A. Biomonitoraggio Della Popolazione Italiana per l’esposizione Ai Metalli: Valori Di Riferimento 1990–2009; (Rapporti ISTISAN 10/22); Istituto Superiore Di Sanità: Rome, Italy, 2010. [Google Scholar]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-156387-1. [Google Scholar]
- Alias, C.; Benassi, L.; Bertazzi, L.; Sorlini, S.; Volta, M.; Gelatti, U. Environmental Exposure and Health Effects in a Highly Polluted Area of Northern Italy: A Narrative Review. Env. Sci. Pollut. Res. 2019, 26, 4555–4569. [Google Scholar] [CrossRef]
- Pavilonis, B.T.; Lioy, P.J.; Guazzetti, S.; Bostick, B.C.; Donna, F.; Peli, M.; Zimmerman, N.J.; Bertrand, P.; Lucas, E.; Smith, D.R.; et al. Manganese Concentrations in Soil and Settled Dust in an Area with Historic Ferroalloy Production. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 443–450. [Google Scholar] [CrossRef]
- Ferri, R.; Hashim, D.; Smith, D.R.; Guazzetti, S.; Donna, F.; Ferretti, E.; Curatolo, M.; Moneta, C.; Beone, G.M.; Lucchini, R.G. Metal Contamination of Home Garden Soils and Cultivated Vegetables in the Province of Brescia, Italy: Implications for Human Exposure. Sci. Total Environ. 2015, 518–519, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Zacco, A.; Resola, S.; Lucchini, R.; Albini, E.; Zimmerman, N.; Guazzetti, S.; Bontempi, E. Analysis of Settled Dust with X-Ray Fluorescence for Exposure Assessment of Metals in the Province of Brescia, Italy. J. Environ. Monit. 2009, 11, 1579–1585. [Google Scholar] [CrossRef]
- Albanese, S.; De Luca, M.L.; De Vivo, B.; Lima, A.; Grezzi, G. Chapter Sixteen—Relationships between Heavy Metal Distribution and Cancer Mortality Rates in the Campania Region, Italy. In Environmental Geochemistry; De Vivo, B., Belkin, H.E., Lima, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 387–400. ISBN 978-0-444-53159-9. [Google Scholar]
- Barba, M.; Mazza, A.; Guerriero, C.; Maio, M.D.; Romeo, F.; Maranta, P.; Marino, I.R.; Paggi, M.G.; Giordano, A. Wasting Lives: The Effects of Toxic Waste Exposure on Health. The Case of Campania, Southern Italy. Cancer Biol. Ther. 2011, 12, 106–111. [Google Scholar] [CrossRef]
- De Felip, E.; Bianchi, F.; Bove, C.; Cori, L.; D’Argenzio, A.; D’Orsi, G.; Fusco, M.; Miniero, R.; Ortolani, R.; Palombino, R.; et al. Priority Persistent Contaminants in People Dwelling in Critical Areas of Campania Region, Italy (SEBIOREC Biomonitoring Study). Sci. Total Environ. 2014, 487, 420–435. [Google Scholar] [CrossRef]
- Mazza, A.; Piscitelli, P.; Falco, A.; Santoro, M.L.; Colangelo, M.; Imbriani, G.; Idolo, A.; De Donno, A.; Iannuzzi, L.; Colao, A. Heavy Environmental Pressure in Campania and Other Italian Regions: A Short Review of Available Evidence. Int. J. Environ. Res. Public Health 2018, 15, 105. [Google Scholar] [CrossRef]
- Cubadda, F.; D’Amato, M.; Aureli, F.; Raggi, A.; Mantovani, A. Dietary Exposure of the Italian Population to Inorganic Arsenic: The 2012–2014 Total Diet Study. Food Chem. Toxicol. 2016, 98, 148–158. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Major and Trace Elements in Tap Water from Italy. J. Geochem. Explor. 2012, 112, 54–75. [Google Scholar] [CrossRef]
- Albanese, S.; De Vivo, B.; Lima, A.; Cicchella, D. Geochemical Background and Baseline Values of Toxic Elements in Stream Sediments of Campania Region (Italy). J. Geochem. Explor. 2007, 93, 21–34. [Google Scholar] [CrossRef]
- Salute Della Popolazione Della Valle Del Sacco. Available online: http://www.deplazio.net/it/salute-della-popolazione-della-valle-del-sacco (accessed on 11 September 2020).
- D’Ilio, S.; Forastiere, F.; Draicchio, A.; Majorani, C.; Petrucci, F.; Violante, N.; Senofonte, O. Human Biomonitoring for Cd, Hg and Pb in Blood of Inhabitants of the Sacco Valley (Italy). Ann. Ist. Super. Sanita 2013, 49, 24–33. [Google Scholar] [CrossRef]
- IBS. Residui, Additivi e Contaminanti Degli Alimenti—Giuseppe Cerutti—Libro—Tecniche Nuove—Tecnica Alimentare. Available online: https://www.ibs.it/residui-additivi-contaminanti-degli-alimenti-libro-giuseppe-cerutti/e/9788848117647 (accessed on 20 December 2020).
- Pizent, A.; Tariba, B.; Živković, T. Reproductive Toxicity of Metals in Men. Arh. Hig. Rada Toksikol. 2012, 63 (Suppl. 1), 35–46. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Public Health Service. Agency for Toxic Substances and Disease Registry Toxicological Profile for Barium and Barium Compounds; ATSDR: Atlanta, Georgia, 2007.
- Kravchenko, J.; Darrah, T.H.; Miller, R.K.; Lyerly, H.K.; Vengosh, A. A Review of the Health Impacts of Barium from Natural and Anthropogenic Exposure. Env. Geochem. Health 2014, 36, 797–814. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Tipton, I.H.; Nason, A.P. Trace Metals in Man: Strontium and Barium. J. Chronic Dis. 1972, 25, 491–517. [Google Scholar] [CrossRef]
- Choudhury, H.; Cary, R.; World Health Organization; International Programme on Chemical Safety. Barium and Barium Compounds; World Health Organization: Geneva, Switzerland, 2001; ISBN 978-92-4-153033-0. [Google Scholar]
- Curtiss, L.F.; Evans, R.D.; De Juren, J.A.; Curtis, H.J.; Epstein, S. Committee on Nuclear Science; USDOE: Oak Ridge, Tennessee, 1960; p. 129. [Google Scholar]
- Li, Y.; Ye, F.; Wang, A.; Wang, D.; Yang, B.; Zheng, Q.; Sun, G.; Gao, X. Chronic Arsenic Poisoning Probably Caused by Arsenic-Based Pesticides: Findings from an Investigation Study of a Household. Int. J. Env. Res. Public Health 2016, 13, 133. [Google Scholar] [CrossRef]
- Historic Arsenical Pesticide Research. 2004. Available online: https://semspub.epa.gov/work/05/259803.pdf (accessed on 11 September 2022).
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of Formulants and Heavy Metals in Glyphosate-Based Herbicides and Other Pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Barenys, M.; Boix, N.; Farran-Codina, A.; Palma-Linares, I.; Montserrat, R.; Curto, A.; Gomez-Catalan, J.; Ortiz, P.; Deza, N.; Llobet, J.M. Heavy Metal and Metalloids Intake Risk Assessment in the Diet of a Rural Population Living near a Gold Mine in the Peruvian Andes (Cajamarca). Food Chem. Toxicol. 2014, 71, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-G.; Chun, O.K.; Song, W.O. Determinants of the Blood Lead Level of US Women of Reproductive Age. J. Am. Coll. Nutr. 2005, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.G. Chelating Agents and Biovailability of Minerals. Nutr. Res. 1981, 1, 617–622. [Google Scholar] [CrossRef]
- Singh, P.; Prasad, S. Spectroscopic Review of Chelating Agents and Their Influence on the Bioavailability of Fe, Zn and Ca in Fijian Foods. Appl. Spectrosc. Rev. 2020, 55, 574–592. [Google Scholar] [CrossRef]
- Szklarska, D.; Rzymski, P. Is Lithium a Micronutrient? From Biological Activity and Epidemiological Observation to Food Fortification. Biol. Trace Elem. Res. 2019, 189, 18–27. [Google Scholar] [CrossRef]
- Naeem, A.; Aslam, M.; Saifullah; Mühling, K.H. Lithium: Perspectives of Nutritional Beneficence, Dietary Intake, Biogeochemistry, and Biofortification of Vegetables and Mushrooms. Sci. Total Environ. 2021, 798, 149249. [Google Scholar] [CrossRef]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Harchegani, A.B. An Overview on Role of Some Trace Elements in Human Reproductive Health, Sperm Function and Fertilization Process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef]
- Sundaram, V.; Srinivas, M.; Gurunathan, J.; Rao, K.; Maniyan, R.P.; Balasundaram, S. Influence of Trace Elements and Their Correlation with Semen Quality in Fertile and Infertile Subjects. Turk. J. Med. Sci. 2013, 43, 1000–1007. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, J.-M. Arsenic Toxicity in Male Reproduction and Development. Available online: http://www.ksdb.org/archive/view_article?pid=dr-19-4-167 (accessed on 18 December 2020).
- Sukhn, C.; Awwad, J.; Ghantous, A.; Zaatari, G. Associations of Semen Quality with Non-Essential Heavy Metals in Blood and Seminal Fluid: Data from the Environment and Male Infertility (EMI) Study in Lebanon. J. Assist. Reprod. Genet. 2018, 35, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, M.A.; Hassan, N.H.A.; Farghaly, A.A.; Hassan, E.E.S. Studies on the Genotoxic Effect of Beryllium Chloride and the Possible Protective Role of Selenium/Vitamins A, C and E. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2008, 652, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yu, G.; Zhang, Y.; Liu, X.; Du, C.; Wang, L.; Li, Z.; Wang, C. Heavy Metal Level in Human Semen with Different Fertility: A Meta-Analysis. Biol. Trace Elem. Res. 2017, 176, 27–36. [Google Scholar] [CrossRef]
- Ahmadi, R.; Faraji, N. In Vitro Effects of Lithium on Human Sperm Motility. In Proceedings of the International Institute of Chemical, Biological & Environmental Engineering, Istanbul, Turkey, 5–6 June 2015. [Google Scholar]
- Bjørklund, G.; Chirumbolo, S.; Dadar, M.; Pivina, L.; Lindh, U.; Butnariu, M.; Aaseth, J. Mercury Exposure and Its Effects on Fertility and Pregnancy Outcome. Basic Clin. Pharmacol. Toxicol. 2019, 125, 317–327. [Google Scholar] [CrossRef]
- Martinez, C.S.; Escobar, A.G.; Torres, J.G.D.; Brum, D.S.; Santos, F.W.; Alonso, M.J.; Salaices, M.; Vassallo, D.V.; Peçanha, F.M.; Leivas, F.G.; et al. Chronic Exposure to Low Doses of Mercury Impairs Sperm Quality and Induces Oxidative Stress in Rats. J. Toxicol. Env. Health A 2014, 77, 143–154. [Google Scholar] [CrossRef]
- Bian, J.; Shi, X.; Li, Q.; Zhao, M.; Wang, L.; Lee, J.; Tao, M.; Wu, X. A Novel Functional Role of Nickel in Sperm Motility and Eukaryotic Cell Growth. J. Trace Elem. Med. Biol. 2019, 54, 142–149. [Google Scholar] [CrossRef]
- Bae, J.-W.; Im, H.; Hwang, J.-M.; Kim, S.-H.; Ma, L.; Kwon, H.J.; Kim, E.; Kim, M.O.; Kwon, W.-S. Vanadium Adversely Affects Sperm Motility and Capacitation Status via Protein Kinase A Activity and Tyrosine Phosphorylation. Reprod. Toxicol. 2020, 96, 195–201. [Google Scholar] [CrossRef]
- Okada, K.; Palmieri, C.; Della Salda, L.; Vackova, I. Viability, Acrosome Morphology and Fertilizing Capacity of Boar Spermatozoa Treated with Strontium Chloride. Zygote 2008, 16, 49–56. [Google Scholar] [CrossRef]
- Nenkova, G.; Petrov, L.; Alexandrova, A. Role of Trace Elements for Oxidative Status and Quality of Human Sperm. Balk. Med. J. 2017, 34, 343–348. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Behnampour, N.; Nejabat, M.; Tabandeh, A.; Ghazi-Moghaddam, B.; Joshaghani, H.R. Impact of Seminal Plasma Trace Elements on Human Sperm Motility Parameters. Rom. J. Intern. Med. 2018, 56, 15–20. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc Levels in Seminal Plasma and Their Correlation with Male Infertility: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yuan, G.; Zhou, Q.; Liu, Y.; He, X.; Zhang, H.; Guo, Y.; Wen, Y.; Huang, S.; Ke, Y.; et al. The Association between Metal Exposure and Semen Quality in Chinese Males: The Mediating Effect of Androgens. Environ. Pollut. 2020, 264, 113975. [Google Scholar] [CrossRef] [PubMed]
- Yuyan, L.; Junqing, W.; Wei, Y.; Weijin, Z.; Ersheng, G. Are Serum Zinc and Copper Levels Related to Semen Quality? Fertil. Steril. 2008, 89, 1008–1011. [Google Scholar] [CrossRef]
- Bal, W.; Jeżowska-Bojczuk, M.; Kasprzak, K.S. Binding of Nickel(II) and Copper(II) to the N-Terminal Sequence of Human Protamine HP2. Chem. Res. Toxicol. 1997, 10, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Bal, W.; Lukszo, J.; Kasprzak, K.S. Mediation of Oxidative DNA Damage by Nickel(II) and Copper(II) Complexes with the N-Terminal Sequence of Human Protamine HP2. Chem. Res. Toxicol. 1997, 10, 915–921. [Google Scholar] [CrossRef]
- Rubio-Riquelme, N.; Huerta-Retamal, N.; Gómez-Torres, M.J.; Martínez-Espinosa, R.M. Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview. Antioxidants 2020, 9, 78. [Google Scholar] [CrossRef]
- Verze, P.; Cai, T.; Lorenzetti, S. The Role of the Prostate in Male Fertility, Health and Disease. Nat. Rev. Urol. 2016, 13, 379–386. [Google Scholar] [CrossRef] [Green Version]


| WRC | BSC | LF | SRV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Range | n | Median | Range | n | Median | Range | n | Median | Range | p Value 1 | |
| Blood serum | |||||||||||||
| Ca | 330 | 157 | 52.4–4627 | 144 | 102 | 52.4–251 | 135 | 184 | 116–4627 | 51 | 188 | 145–3082 | 0.0001 |
| Mg | 330 | 29.8 | 10.7–135 | 144 | 20.1 | 10.7–44.1 | 135 | 32.1 | 22.7–119 | 51 | 41 | 33.4–135 | 0.0001 |
| Na | 256 | 4455 | 3238–10,449 | 70 | 4322 | 3238–6399 | 135 | 4471 | 3485–7383 | 51 | 4576 | 4115–10,449 | 0.0001 |
| K | 256 | 229 | 123–878 | 70 | 210 | 123–278 | 135 | 231 | 162–878 | 51 | 250 | 200–570 | 0.0001 |
| Semen | |||||||||||||
| Ca | 262 | 488 | 117–1193 | 113 | 560 | 237–1193 | 94 | 453 | 133–1171 | 55 | 412 | 117–773 | 0.0001 |
| Mg | 262 | 128 | 11.6–449 | 113 | 172 | 25.6–441 | 94 | 120 | 11.6–449 | 55 | 103 | 19.7–251 | 0.0001 |
| Na | 262 | 4302 | 91.9–9745 | 113 | 4915 | 2397–9745 | 94 | 3960 | 91.9–7777 | 55 | 3900 | 354–6759 | 0.0001 |
| K | 262 | 1866 | 12.7–3879 | 113 | 2063 | 1074–3879 | 94 | 1712 | 12.7–3464 | 55 | 1632 | 145–3508 | 0.0001 |
| WRC | BSC | LF | SRV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Range | n | Median | Range | n | Median | Range | n | Median | Range | p Value 1 | |
| Blood serum | |||||||||||||
| Cu | 332 | 842 | 445–2049 | 144 | 786 | 461–2049 | 137 | 918 | 554–1807 | 51 | 766 | 445–1103 | 0.0001 |
| Mn | 286 | 4.8 | 0.4–35.9 | 124 | 3.9 | 0.4–19.5 | 137 | 6.4 | 1.4–35.9 | 25 | 3.1 | 0.8–13.1 | 0.0001 |
| Se | 332 | 103 | 50.3–248 | 144 | 98.4 | 66.5–248 | 137 | 110 | 85.7–162 | 51 | 91.1 | 50.3–122 | 0.0001 |
| Zn | 331 | 1204 | 618–4820 | 144 | 1285 | 736–4820 | 136 | 1214 | 710–2026 | 51 | 884 | 618–1358 | 0.0001 |
| Semen | |||||||||||||
| Cu | 268 | 142 | 36.9–1085 | 113 | 165 | 76.6–635 | 100 | 144 | 44.1–1085 | 55 | 118 | 36.9–482 | 0.0001 |
| Fe | 268 | 1375 | 329–119,401 | 113 | 2662 | 428–47,180 | 100 | 1166 | 329–119,401 | 55 | 807 | 375–88,138 | 0.0001 |
| Mn | 268 | 10.1 | 2.5–133 | 113 | 12.8 | 3.3–108 | 100 | 8.9 | 2.5–133 | 55 | 7.9 | 2.7–50.6 | 0.0033 |
| Ni | 152 | 14.4 | 4.4–240 | 109 | 27.1 | 5.5–240 | 100 | <4.2 | - | 43 | 7.5 | 4.5–66.8 | - |
| Se | 268 | 38.2 | 3.9–119 | 113 | 49.4 | 18.4–110 | 100 | 36.5 | 6.6–119 | 55 | 36.5 | 6.6–119 | 0.0001 |
| Zn | 268 | 91,316 | 48–526,312 | 113 | 130,430 | 400–526,312 | 100 | 59,137 | 48–219,654 | 55 | 68,206 | 200–223,536 | 0.0001 |
| WRC | BSC | LF | SRV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Range | n | Median | Range | n | Median | Range | n | Median | Range | p Value 1 | |
| Blood serum | |||||||||||||
| As | 332 | 2.9 | 0.2–97.9 | 144 | 4.8 | 0.3–36.9 | 137 | 2.3 | 0.7–97.9 | 51 | 1.5 | 0.2–13.7 | 0.0001 |
| Ba | 261 | 18.0 | 6.7–1268 | 129 | 25.6 | 6.7–1268 | 103 | 12.1 | 6.8–145 | 29 | 10.8 | 6.8–27.4 | 0.0001 |
| Cd | 105 | 0.7 | 0.4–2.7 | 144 | <0.2 | - | 86 | 0.7 | 0.4–2.7 | 19 | 0.4 | 0.2–1.3 | - |
| Hg | 144 | 0.7 | 0.2–4.4 | 95 | 0.6 | 0.2–2.9 | 88 | 0.9 | 0.2–4.4 | 51 | <0.2 | - | - |
| Li | 317 | 12.5 | 0.7–371 | 136 | 30.3 | 0.7 – 106 | 132 | 11.6 | 1.8–22.3 | 49 | 13 | 8.3–371 | 0.0295 |
| Pb | 318 | 1.3 | 0.1–231 | 136 | 1.9 | 0.2–231 | 131 | 1.2 | 0.1–40.5 | 51 | 1 | 0.2–3.9 | 0.0001 |
| Rb | 332 | 159 | 84.2–1093 | 144 | 141 | 89.4–334 | 137 | 162 | 91.3–1093 | 51 | 194 | 102–289 | 0.0001 |
| Sb | 292 | 1.1 | 0.2–7.6 | 137 | 2 | 0.3–7.6 | 113 | 0.9 | 0.3–6.7 | 42 | 0.7 | 0.2–7.2 | 0.0001 |
| Sn | 281 | 1.9 | 0.2–36.9 | 96 | 1.2 | 0.2–36.9 | 136 | 2.3 | 0.2 – 5.1 | 49 | 1.8 | 0.2–3.6 | 0.0001 |
| Sr | 332 | 30.8 | 14.5–124 | 144 | 32.3 | 15.1–124 | 137 | 28.7 | 14.5–60.3 | 51 | 32.8 | 14.7–56.0 | 0.0019 |
| U | 193 | 0.3 | 0.2–2.5 | 72 | 0.3 | 0.2–2.5 | 96 | 0.3 | 0.2–1.5 | 25 | 0.3 | 0.2–0.7 | 0.0764 |
| Semen | |||||||||||||
| As | 267 | 4.6 | 0.2–33.7 | 112 | 4.0 | 0.2–33.7 | 100 | 6.0 | 1.6–17.8 | 55 | 4.0 | 1.0–16.5 | 0.0001 |
| Ba | 204 | 74.5 | 26.0–19,847 | 49 | 48.4 | 26.0–103 | 100 | 75.2 | 27.6–3177 | 55 | 128.2 | 48.3–19,847 | 0.0001 |
| Hg | 120 | 0.5 | 0.2–2.7 | 39 | 0.7 | 0.2–1.8 | 81 | 0.5 | 0.2–2.7 | 54 | <0.2 | - | - |
| Li | 267 | 27.5 | 0.4–210 | 112 | 24.2 | 0.4–43.5 | 100 | 102 | 1.4–210 | 55 | 24.2 | 5.2–35.5 | 0.0001 |
| Pb | 257 | 2.4 | 0.1–48.7 | 102 | 1.6 | 0.1–11.5 | 100 | 3.5 | 0.4–22.4 | 55 | 2.4 | 0.5–48.7 | 0.0001 |
| Rb | 268 | 1489 | 214–4847 | 113 | 1504 | 688–2717 | 100 | 1315 | 328–3053 | 55 | 1733 | 214–4847 | 0.0025 |
| Sb | 100 | 3.9 | 0.2–79.4 | 66 | 4.1 | 0.2–14.1 | 34 | 3.1 | 0.3–79.4 | 55 | <0.2 | - | - |
| Sn | 260 | 3.5 | 0.2–28.4 | 112 | 3.5 | 3.1–27.4 | 94 | 3.5 | 2.4–28.4 | 54 | 2.3 | 3.6–8.8 | 0.0001 |
| Sr | 268 | 70.8 | 206–272 | 113 | 76.1 | 32.3–272 | 100 | 67.0 | 22.3–186 | 55 | 67.6 | 20.6–152 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunzio, A.D.; Giarra, A.; Toscanesi, M.; Amoresano, A.; Piscopo, M.; Ceretti, E.; Zani, C.; Lorenzetti, S.; Trifuoggi, M.; Montano, L. Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy. Int. J. Environ. Res. Public Health 2022, 19, 11635. https://doi.org/10.3390/ijerph191811635
Nunzio AD, Giarra A, Toscanesi M, Amoresano A, Piscopo M, Ceretti E, Zani C, Lorenzetti S, Trifuoggi M, Montano L. Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy. International Journal of Environmental Research and Public Health. 2022; 19(18):11635. https://doi.org/10.3390/ijerph191811635
Chicago/Turabian StyleNunzio, Aldo Di, Antonella Giarra, Maria Toscanesi, Angela Amoresano, Marina Piscopo, Elisabetta Ceretti, Claudia Zani, Stefano Lorenzetti, Marco Trifuoggi, and Luigi Montano. 2022. "Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy" International Journal of Environmental Research and Public Health 19, no. 18: 11635. https://doi.org/10.3390/ijerph191811635