Making Pb Adsorption-Saturated Attapulgite with Excellent Photocatalysis Properties through a Vulcanization Reaction and Its Application for MB Wastewater Degradation
Abstract
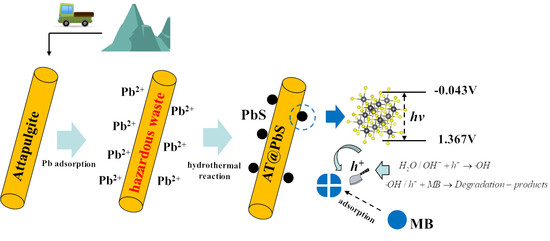
:1. Introduction
2. Experiment and Methods
2.1. Synthesis of Catalyst
2.2. Materials Characterization
2.3. Photocatalytic Experiments
3. Results and Discussion
3.1. XRD
3.2. UV-Vis Diffuse Reflectance Spectra
3.3. XPS
3.4. TEM
3.5. Photocatalytic Performance
3.6. The Photocatalytic Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vu, H.H.T.; Gu, S.; Thriveni, T.; Khan, M.D.; Tuan, L.Q.; Ahn, J.W. Sustainable Treatment for Sulfate and Lead Removal from Battery Wastewater. Sustainability 2019, 11, 3497. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Nafees, M.; Ge, L.; Khan, M.H.; Bilal, M.; Chan, W.P.; Lisak, G. Assessment of industrial wastewater for potentially toxic elements, human health (dermal) risks, and pollution sources: A case study of Gadoon Amazai industrial estate, Swabi, Pakistan. J. Hazard. Mater. 2021, 419, 126450. [Google Scholar] [CrossRef] [PubMed]
- Relić, D.; Sakan, S.; Anđelković, I.; Popović, A.; Đorđević, D. Pollution and Health Risk Assessments of Potentially Toxic Elements in Soil and Sediment Samples in a Petrochemical Industry and Surrounding Area. Molecules 2019, 24, 2139. [Google Scholar] [CrossRef] [Green Version]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.N.; Mohanty, C.; Meikap, B. Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Chen, C.; Tang, R.; Han, C.H.; Tian, Y. Competitive Adsorption of Cu, Ni, Pb, and Cd from Aqueous Solution onto Fly Ash-Based Linde F(K) Zeolite. Iran. J. Chem. Chem. Eng. 2018, 37, 61–72. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, T.; Zhang, X.; Wu, R.; Wang, Q. Synthesis of an Efficient Pb Adsorption Nano-Crystal under Strong Alkali Hydrothermal Environment Using a Gemini Surfactant as Directing Agent. J. Chem. Soc. Pak. 2019, 41, 1034–1038. [Google Scholar] [CrossRef]
- Awual, M.R. Innovative composite material for efficient and highly selective Pb(II) ion capturing from wastewater. J. Mol. Liq. 2019, 284, 502–510. [Google Scholar] [CrossRef]
- Awual, M.R. Novel conjugated hybrid material for efficient lead(II) capturing from contaminated wastewater. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 101, 686–695. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Chen, Y.; Li, Y.; Zhang, R.; Tang, C.; Hu, X. Cd(II) and Pb(II) absorbed on humic acid-iron-pillared bentonite: Kinetics, thermodynamics and mechanism of adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126005. [Google Scholar] [CrossRef]
- Huang, R.; Lin, Q.; Zhong, Q.; Zhang, X.; Wen, X.; Luo, H. Removal of Cd(II) and Pb(II) from aqueous solution by modified attapulgite clay. Arab. J. Chem. 2020, 13, 4994–5008. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wang, J.; Tang, Y.; Zhang, Z. Selective adsorption of Pb2+ and Cu2+ on amino-modified attapulgite: Kinetic, thermal dynamic and DFT studies. J. Hazard. Mater. 2021, 404 Pt A, 124140. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2018, 17, 629–654. [Google Scholar] [CrossRef]
- Pyrgaki, K.; Messini, P.; Zotiadis, V. Adsorption of Pb and Cu from Aqueous Solutions by Raw and Heat-Treated Attapulgite Clay. Geosciences 2018, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, S.K.; Paramasivan, M.; Al-Mukhtar, M.; Tiyasha, T.; Pyrgaki, K.; Tung, T.M.; Yaseen, Z.M. Prediction of lead (Pb) adsorption on attapulgite clay using the feasibility of data intelligence models. Environ. Sci. Pollut. Res. 2021, 28, 31670–31688. [Google Scholar] [CrossRef]
- Chhabra, V.A.; Kaur, R.; Walia, M.S.; Kim, K.-H.; Deep, A. PANI/PbS QD nanocomposite structure for visible light driven photocatalytic degradation of rhodamine 6G. Environ. Res. 2020, 186, 109615. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Li, Y.; Wang, Y.; Liu, X.; Liu, C.; Luo, S. Reduced graphene oxide and PbS nanoparticles co-modified TiO2 nanotube arrays as a recyclable and stable photocatalyst for efficient degradation of pentachlorophenol. Appl. Catal. A Gen. 2013, 457, 78–84. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Zhang, Y.; Xing, X.; Li, H. High Photocatalytic Activity of g-C3N4/La-N-TiO2 Composite with Nanoscale Heterojunctions for Degradation of Ciprofloxacin. Int. J. Environ. Res. Public Health 2022, 19, 4793. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Ding, N. Visible Light-Based Ag3PO4/g-C3N4@MoS2 for Highly Efficient Degradation of 2-Amino-4-acetylaminoanisole (AMA) from Printing and Dyeing Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 2934. [Google Scholar] [CrossRef]
- Lin, H.; Yang, Y.; Shang, Z.; Li, Q.; Niu, X.; Ma, Y.; Liu, A. Study on the Enhanced Remediation of Petroleum-Contaminated Soil by Biochar/g-C3N4 Composites. Int. J. Environ. Res. Public Health 2022, 19, 8290. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, P.A.; Oluwalana, A.E. Structural, Optical, Photocatalytic and Electrochemical Studies of PbS Nanoparticles. J. Nano Res. 2020, 61, 18–31. [Google Scholar] [CrossRef]
- Sehati, S.; Entezari, M. Entezari. High visible light intercalated nanophotocatalyst (PbS-CdS/Ti6O13) synthesized by ultrasound: Photocatalytic activity, photocorrosion resistance and degradation mechanism. Sep. Purif. Technol. 2017, 174, 482–492. [Google Scholar] [CrossRef]
- Raja, V.R.; Rosaline, D.R.; Suganthi, A.; Rajarajan, M. Facile fabrication of PbS/MoS2 nanocomposite photocatalyst with efficient photocatalytic activity under visible light. Solid State Sci. 2017, 67, 99–108. [Google Scholar] [CrossRef]
- Lv, Y.-R.; Huo, R.; Yang, S.; Liu, Y.-Q.; Li, X.-J.; Xu, Y.-H. Self-assembled synthesis of PbS quantum dots supported on polydopamine encapsulated BiVO4 for enhanced visible-light-driven photocatalysis. Sep. Purif. Technol. 2018, 197, 281–288. [Google Scholar] [CrossRef]
- Kar, A.; Sain, S.; Rossouw, D.; Knappett, B.R.; Pradhan, S.K.; Wheatley, A.E.H. Facile synthesis of SnO2-PbS nanocomposites with controlled structure for applications in photocatalysis. Nanoscale 2016, 8, 2727–2739. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Fan, W.; Liu, Z.; Dong, H.; Yan, Y.; Huo, P. Construction of an attapulgite intercalated mesoporous g-C3N4 with enhanced photocatalytic activity for antibiotic degradation. J. Photochem. Photobiol. A Chem. 2018, 359, 102–110. [Google Scholar] [CrossRef]
- Zuo, S.; Chen, Y.; Liu, W.; Yao, C.; Li, X.; Li, Z.; Ni, C.; Liu, X. A facile and novel construction of attapulgite/Cu2O/Cu/g-C3N4 with enhanced photocatalytic activity for antibiotic degradation. Ceram. Int. 2017, 43, 3324–3329. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Lu, X.; Zuo, S.; Yao, C.; Ni, C. Integrated nanostructures of CeO2/attapulgite/g-C3N4 as efficient catalyst for photocatalytic desulfurization: Mechanism, kinetics and influencing factors. Chem. Eng. J. 2017, 326, 87–98. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, T.; Wang, L.; Xu, Y.; Zhang, X. Surface functionalization of Linde F (K) nano-zeolite and its application for photocatalytic wastewater treatment and hydrogen production. Appl. Phys. A 2022, 128, 468. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Cheng, T.; Zhang, X.; Zhou, Z.; Zhang, X.; Xu, Q. Ag3PO4/AgSbO3 composite as novel photocatalyst with significantly enhanced activity through a Z-scheme degradation mechanism. J. Iran. Chem. Soc. 2021, 19, 821–838. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, T.; Chen, C.; Wang, L.; Deng, Q.; Chen, G.; Ye, C. Synthesis of a novel magnetic nano-zeolite and its application as an efficient heavy metal adsorbent. Mater. Res. Express 2020, 7, 085007. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, C.; Wang, L.; Zhang, X.; Ye, C.; Deng, Q.; Chen, G. Synthesis of Fly Ash Magnetic Glass Microsphere@BiVO4 and Its Hybrid Action of Visible-Light Photocatalysis and Adsorption Process. Pol. J. Environ. Stud. 2021, 30, 2027–2040. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Cheng, T.; Zhang, X.; Tian, Y.; Shi, Y. Sliver Doped Sodium Antimonate with Greatly Reduced the Band Gap for Efficiently Enhanced Photocatalytic Activities Under Visible Light (Experiment and DFT Calculation). Mater. Res. 2021, 24, e20210100. [Google Scholar] [CrossRef]
- Hou, Z.; Chu, J.; Liu, C.; Wang, J.; Li, A.; Lin, T.; François-Xavier, C.P. High efficient photocatalytic reduction of nitrate to N2 by Core-shell Ag/SiO2@cTiO2 with synergistic effect of light scattering and surface plasmon resonance. Chem. Eng. J. 2021, 415, 128863. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, Y.; Wang, J.; Li, A.; Bian, W.; Corvini, P.F.-X. Au@CoS-BiVO4 {010} Constructed for Visible-Light-Assisted Peroxymonosulfate Activation. Catalysts 2021, 11, 1414. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Wang, J.; François-Xavier, C.P.; Wintgens, T. Highly efficient Z-scheme structured visible-light photocatalyst constructed by selective doping of Ag@AgBr and Co3O4 separately on {010} and {110} facets of BiVO4: Pre-separation channel and hole-sink effects. Appl. Catal. B Environ. 2019, 250, 31–41. [Google Scholar] [CrossRef]
- Guo, X.; Rao, L.; Shi, Z. Preparation of High-Porosity B-TiO2/C3N4 Composite Materials: Adsorption–Degradation Capacity and Photo-Regeneration Properties. Int. J. Environ. Res. Public Health 2022, 19, 8683. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, Y.; Chen, L.; Bian, W.; Wang, J. Pt Nanowire-Anchored Dodecahedral Ag3PO4{110} Constructed for Significant Enhancement of Photocatalytic Activity and Anti-Photocorrosion Properties: Spatial Separation of Charge Carriers and PhotogeneratedElectron Utilization. Catalysts 2020, 10, 206. [Google Scholar] [CrossRef] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, C.; Cheng, T.; Wen, M.; Wang, L.; Pan, F. Making Pb Adsorption-Saturated Attapulgite with Excellent Photocatalysis Properties through a Vulcanization Reaction and Its Application for MB Wastewater Degradation. Int. J. Environ. Res. Public Health 2022, 19, 10457. https://doi.org/10.3390/ijerph191610457
Zhang X, Chen C, Cheng T, Wen M, Wang L, Pan F. Making Pb Adsorption-Saturated Attapulgite with Excellent Photocatalysis Properties through a Vulcanization Reaction and Its Application for MB Wastewater Degradation. International Journal of Environmental Research and Public Health. 2022; 19(16):10457. https://doi.org/10.3390/ijerph191610457
Chicago/Turabian StyleZhang, Xiao, Chen Chen, Ting Cheng, Mingyue Wen, Lei Wang, and Fenxu Pan. 2022. "Making Pb Adsorption-Saturated Attapulgite with Excellent Photocatalysis Properties through a Vulcanization Reaction and Its Application for MB Wastewater Degradation" International Journal of Environmental Research and Public Health 19, no. 16: 10457. https://doi.org/10.3390/ijerph191610457