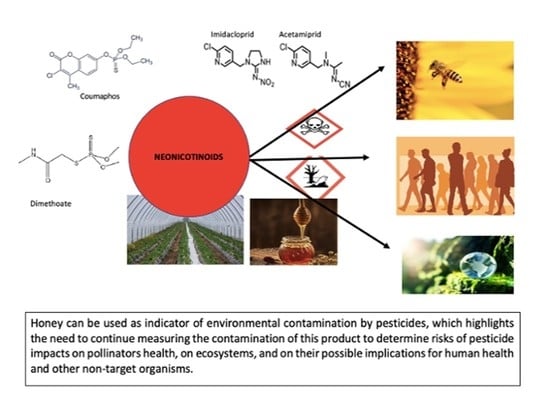
Detection and Concentration of Neonicotinoids and Other Pesticides in Honey from Honey Bee Colonies Located in Regions That Differ in Agricultural Practices: Implications for Human and Bee Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Sample Collection
2.3. Laboratory Analysis of Honey Samples
2.3.1. Identification Criteria, Quality Control, and Validation of the Analytical Procedure
2.3.2. Performance Results of the Analysis Methodology
2.4. Statistical Analyses
3. Results
3.1. Pesticides Detected
3.2. Frequency of Pesticides Detected by Zone
3.3. Pesticide Concentrations
3.4. Pesticide Levels and Risk to Human Health
3.5. Pesticide Levels and Risk to Honey Bee Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations (UN). Desafíos Globales, Población. 2020. Available online: https://www.un.org/es/sections/issues-depth/population/index.html (accessed on 18 July 2020).
- World Health Organization (WHO). Connecting Global Priorities: Biodiversity and Human Health: A State of Knowledge Review. 2015. Available online: http://apps.who.int/iris/bitstream/handle/10665/174012/9789241508537_eng.pdf;jsessionid=9246190A9D78ACDAAF4C35ECBA1E5760?sequence=1 (accessed on 3 February 2021).
- Montiel-González, C.; Gallegos-Tavera, A.; Ortega-Gómez, A.M.; Bautista, F.; Gopar-Merino, F.; Velázquez, A. Análisis climático para la agricultura de temporal en Michoacán, México. Ecosistem. Rec. Agropec. 2019, 6, 307–316. [Google Scholar] [CrossRef]
- Jensen, M.H.; Malter, A.J. Protected Agriculture: A Global Review. World Bank Publications: Washington, DC, USA, 1995. [Google Scholar]
- Mahmoud, F.M.; Loutfy, N. Uses and environmental pollution of biocides. In Pesticides: Evaluation of Environmental Pollution; Rathore, H., Nollet, L., Eds.; Taylor and Francis: New York, NY, USA, 2012; pp. 3–25. [Google Scholar]
- Robson, M.; Hamilton, G.; Siriwong, W. Pest control and pesticides. In Environmental Health from Global to Local; Frumkin, H., Ed.; Jossey-Bass: San Francisco, CA, USA, 2010; pp. 593–632. [Google Scholar]
- Tomizawa, M.; Casida, J.E. Neonicotinoids insecticides: Highlights of a symposium on strategic molecular designs. J. Agric. Food Chem. 2011, 59, 2883–2886. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorgo, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Poll. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Ichikawa, G.; Kuribayashi, R.; Ikenaka, Y.; Ichise, T.; Nakayama, S.M.; Ishizuka, M.; Taira, K.; Fujioka, K.; Sairecnchi, T.; Kobashi, G.; et al. LC-ESI/MS/MS analysis of neonicotinoids in urine of very low birth weight infants at birth. PLoS ONE 2019, 14, e0219208. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Giorio, C.; Sánchez-Bayo, F.; van Lexmond, M.B. An update of the worldwide integrated assessment (WIA) on systemic insecticides. Environ. Sci. Pollut. Res. 2021, 28, 11709–11715. [Google Scholar] [CrossRef] [PubMed]
- Pisa, L.; Goulson, D.; Yang, E.-C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; van der Sluijs, J.; MacQuarrie, C.J.; Giorio, C.; et al. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: Impacts on organisms and ecosystems. Environ. Sci. Poll. Res. 2017, 28, 11749–11797. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef]
- Feng, S.; Kong, Z.; Wang, X.; Peng, P.; Zeng, E.Y. Assessing the genotoxicity of imidacloprid and RH-5849 in human peripheral blood lymphocytes in vitro with comet assay and cytogenetic tests. Ecotoxicol. Environ. Saf. 2005, 61, 239–246. [Google Scholar] [CrossRef]
- Lu, C.; Warchol, K.M.; Callahan, R.A. In Situ replication of honey bee colony collapse disorder. Bull. Insectology 2012, 65, 99–106. [Google Scholar]
- Van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.-M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Underwood, R.; Caron, D.; Hayes, J. Estimate of managed colony losses in the winter of 2006–2007: A report commissioned by the apiary inspectors of America. Am. Bee J. 2007, 147, 599–603. [Google Scholar]
- Van Lexmond, M.B.; Bonmatin, J.-M.; Goulson, D.; Noome, D.A. Worldwide integrated assessment on systemic pesticides. Environ. Sci. Poll. Res. 2015, 22, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman-Novoa, E. Colony collapse disorder and other threats to honey bees. In One Health Case Studies: Addressing Complex Problems in a Changing World, Cork, S., Hall, D., Liljebjelke, K., Eds.; 5M Publishing Ltd.: Sheffield, UK, 2016; pp. 204–216. [Google Scholar]
- Van Engelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Correa-Benitez, A.; Guzman-Novoa, E. Sublethal exposure to clothianidin during the larval stage causes long-term impairment of hygienic and foraging behaviours of honey bees. Apidologie 2019, 50, 595–605. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Hunt, G.J.; Guzman-Novoa, E. Effects of sublethal doses of clothianidin and/or V. destructor on honey bee (Apis mellifera) self-grooming behavior and associated gene expression. Sci. Rep. 2019, 9, 5196. [Google Scholar] [CrossRef] [Green Version]
- Morfin, N.; Goodwin, P.H.; Correa-Benitez, A.; Guzman-Novoa, E. The combined effects of Varroa destructor parasitism and exposure to neonicotinoids affects honey bee (Apis mellifera L.) memory and gene expression. Biology 2020, 9, 237. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of Varroa destructor and sublethal clothianidin doses during the larval stage on subsequent adult honey bee (Apis mellifera L.) health, cellular immunity, deformed wing virus levels and differential gene expression. Microorganisms 2020, 8, 858. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of field realistic doses of clothianidin and Varroa destructor parasitism on adult honey bee (Apis mellifera L.) health and neural gene expression, and antagonistic effects on differentially expressed genes. PLoS ONE 2020, 15, e0229030. [Google Scholar] [CrossRef] [Green Version]
- Tarek, H.; Hamiduzzaman, M.M.; Morfin, N.; Guzman-Novoa, E. Sub-lethal doses of neonicotinoid and carbamate insecticides reduce the lifespan and alter the expression of immune health and detoxification related genes of honey bees (Apis mellifera). Gen. Mol. Res. 2018, 17, gmr16039908. [Google Scholar] [CrossRef]
- Tison, L.; Rößner, A.; Gerschewski, S.; Menzel, R. The neonicotinoid clothianidin impairs memory processing in honey bees. Ecotoxicol. Environ. Saf. 2019, 180, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risk of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porrini, C.; Sabatini, A.G.; Girotti, S.; Fini, F.; Monaco, L.; Celli, G.; Bortolotti, L.; Ghini, S. The death of honey bees and environmental pollution by pesticides: The honey bees as biological indicators. Bull. Insectology 2003, 56, 147–152. [Google Scholar]
- Traynor, K.S.; Tosi, S.; Rennich, K.; Steinhauer, N.; Forsgren, E.; Rose, R.; Kunkel, G.; Madella, S.; Lopez, D.; Eversole, H.; et al. Pesticides in honey bee colonies: Establishing a baseline for real world exposure over seven years in the USA. Environ. Pollut. 2021, 279, 116566. [Google Scholar] [CrossRef] [PubMed]
- Vandame, R. Abejas e insecticidas. In Los Plaguicidas Altamente Peligrosos en México; Bejarano-González, F., Ed.; Red de Acción sobre Plaguicidas y Alternativas en México (RAPAM): Texcoco, México, 2017; pp. 167–185. [Google Scholar]
- Cunningham, M.; Tran, L.; Mckee, C.; Ortega, R.; Newman, T.; Lansing, L.; Griffiths, J.; Bilodeau, G.; Rott, M.; Guarna, M. Honey bees as biomonitors of environmental contaminants, pathogens, and climate change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Rashed, M.N.; El-Haty, M.T.; Mohamed, S.M. Bee honey as environmental indicator for pollution with heavy metals. Toxicol. Environ. Chem. 2009, 91, 389–403. [Google Scholar] [CrossRef]
- Fermo, P.; Beretta, G.; Facino, R.M.; Gelmini, F.; Piazzalunga, A. Ionic profile of honey as potential indicator of botanical origin and global environmental pollution. Environ. Pollut. 2013, 178, 173–181. [Google Scholar] [CrossRef]
- Gilbert, M.D.; Lisk, D.J. Honey as an environmental indicator of radionuclide contamination. Bull. Environ. Cont. Toxicol. 1978, 19, 32–34. [Google Scholar] [CrossRef]
- Mitchell, E.A.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Cernansky, R. Controversial pesticides found in honey samples from six continents. Nature 2017. [Google Scholar] [CrossRef]
- Valdovinos-Flores, C.; Alcantar-Rosales, V.M.; Gaspar-Ramírez, O.; Saldaña-Loza, L.M.; Dorantes-Ugalde, J.A. Agricultural pesticide residues in honey and wax combs from Southeastern and Northeastern Mexico. J. Apic. Res. 2017, 56, 667–679. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística; Geografía e Informática (INEGI). Encuesta Nacional Agropecuaria. Superficie Cultivada y Producción de Cultivos Anuales por Entidad. Producción de Agricultura protegida por entidad. 2018. Available online: https://www.inegi.org.mx/programas/ena/2017/default.html#Tabulados (accessed on 5 August 2020).
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Agricultura de Temporal por Municipio. 2019. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 8 October 2020).
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Anuario Estadístico de Producción Ganadera/miel por Municipio de Jalisco. 2017. Available online: https://nube.siap.gob.mx/cierre_pecuario/ (accessed on 1 February 2020).
- Google Earth. Jalisco State Map, Mexico at Google Maps. Available online: https://www.google.com/maps/place/Jalisco (accessed on 3 March 2021).
- Wang, J.; Leung, D. Determination of 142 pesticides in fruit and vegetable based infant foods by liquid chromatography/electrospray ionization-tandem mass spectrometry and estimation of measurement uncertainty. J. AOAC Int. 2009, 92, 279–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesticide Action Network International (PAN). Pesticide Info. by Chemical Search. 2018. Available online: http://www.pesticideinfo.org (accessed on 7 July 2021).
- European Commission (EC). Maximum Residue Levels. 2018. Available online: https://ec.europa.eu/food/plant/pesticides/max_residue_levels_en (accessed on 2 January 2021).
- Williamson, S.M.; Wright, G.A. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J. Exp. Biol. 2013, 216, 1799–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Mullin, C.A.; Chen, J.; Fine, J.D.; Frazier, M.T.; Frazier, J.L. The formulation makes the honey bee poison. Pestic. Biochem. Physiol. 2015, 120, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Giroud, B.; Jabot, C.; Vulliet, E. Exposure assessment of honeybees through study of hive matrices: Analysis of selected pesticide residues in honeybees, beebread, and beeswax from French beehives by LC-MS/MS. Environ. Sci. Pollut Res. 2018, 25, 6145–6153. [Google Scholar] [CrossRef]
- Scripcâ, L.A.; Amariei, S. The Influence of Chemical Contaminants on the Physicochemical Properties of Unifloral and Multifloral Honey. Foods 2021, 10, 1039. [Google Scholar] [CrossRef]
- Eckert, J. The flight range of the honeybee. J. Agric. Res. 1933, 47, 257–285. [Google Scholar]
- Morse, R. Research review. How far will bees fly? Glean. Bee Cult. 1984, 112, 474. [Google Scholar]
- Herrero-LaTorre, C.; Barciela-García, J.; García-Martín, S.; Crescente-Peña, R.M. The Use of Honeybees and Honey as Environmental Bioindicators for Metals and Radionuclides: A Review. 2017. Available online: https://tspace.library.utoronto.ca/bitstream/1807/78891/1/er-2017-0029.pdf (accessed on 15 January 2021).
- Pesticide Action Network International (PAN). PAN International List of Highly Hazardous Pesticides. 2015. Available online: https://www.uccs.mx/images/library/file/externos/HHP_Lista_PAN_2015corr.pdf (accessed on 7 December 2021).
- Caron-Beaudoin, É.; Viau, R.; Sanderson, J.T. Effects of neonicotinoid pesticides on promoter-specific aromatase (CYP19) expression in Hs578t breast cancer cells and the role of the VEGF pathway. Environ. Health Persp. 2018, 126, 047014. [Google Scholar] [CrossRef]
- Yang, E.C.; Chang, H.C.; Wu, W.Y.; Chen, Y.-W. Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 2012, 7, e49472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Eiri, D.M.; Nieh, J.C. A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. J. Experim. Biol. 2012, 215, 2022–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.W.; Tautz, J.; Grünewald, B.; Fuchs, S. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE 2012, 7, e30023. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Jalisco’s Zone | Compound | Chemical Family | Use | Number of Samples with Pesticide | Number of Samples without Pesticide | Mean Concentration ± SE a |
|---|---|---|---|---|---|---|
| North | imidacloprid | neonicotinoid | Insecticide | 2 | 14 | 0.37 ± 0.13 |
| metadimophos | organophosphate | Insecticide | 1 | 15 | 0.2 ± 0 | |
| coumaphos | organophosphate | Insecticide | 3 | 13 | 0.15 ± 0.06 | |
| monocrotophos | organophosphate | Insecticide | 1 | 15 | 0.26 ± 0 | |
| dimethoate | organophosphate | Insecticide | 3 | 13 | 0.32 ± 0.09 | |
| omethoate | organophosphate | Insecticide | 2 | 14 | 0.24 ± 0.07 | |
| acephate | organophosphate | Insecticide | 1 | 15 | 0.83 ± 0 | |
| carbendazim | benzimidazole | Fungicide | 1 | 15 | 0.12 ± 0 | |
| diuron | urea | Herbicide | 1 | 15 | 0.19 ± 0 | |
| South | imidacloprid | neonicotinoid | Insecticide | 6 | 8 | 1.16 ± 0.42 |
| acetamiprid | neonicotinoid | Insecticide | 4 | 10 | 7.55 ± 5.19 | |
| formetanate | methyl-carbamate | Insecticide | 1 | 13 | 26 ± 0 | |
| metadimophos | organophosphate | Insecticide | 3 | 11 | 1.02 ± 0.42 | |
| coumaphos | organophosphate | Insecticide | 5 | 9 | 0.52 ± 0.30 | |
| omethoate | organophosphate | Insecticide | 1 | 13 | 0.12 ± 0 | |
| propamocarb | carbamate | Fungicide | 3 | 11 | 0.26 ± 0.04 | |
| carbendazim | benzimidazole | Fungicide | 3 | 11 | 0.36 ± 0.17 | |
| boscalid | anilide | Fungicide | 1 | 13 | 0.34 ± 0 | |
| fenhexamid | anilide | Fungicide | 1 | 13 | 0.39 ± 0 | |
| diuron | urea | Herbicide | 2 | 12 | 0.48 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce-Vejar, G.; Ramos de Robles, S.L.; Macias-Macias, J.O.; Petukhova, T.; Guzman-Novoa, E. Detection and Concentration of Neonicotinoids and Other Pesticides in Honey from Honey Bee Colonies Located in Regions That Differ in Agricultural Practices: Implications for Human and Bee Health. Int. J. Environ. Res. Public Health 2022, 19, 8199. https://doi.org/10.3390/ijerph19138199
Ponce-Vejar G, Ramos de Robles SL, Macias-Macias JO, Petukhova T, Guzman-Novoa E. Detection and Concentration of Neonicotinoids and Other Pesticides in Honey from Honey Bee Colonies Located in Regions That Differ in Agricultural Practices: Implications for Human and Bee Health. International Journal of Environmental Research and Public Health. 2022; 19(13):8199. https://doi.org/10.3390/ijerph19138199
Chicago/Turabian StylePonce-Vejar, Gilda, S. Lizette Ramos de Robles, José Octavio Macias-Macias, Tatiana Petukhova, and Ernesto Guzman-Novoa. 2022. "Detection and Concentration of Neonicotinoids and Other Pesticides in Honey from Honey Bee Colonies Located in Regions That Differ in Agricultural Practices: Implications for Human and Bee Health" International Journal of Environmental Research and Public Health 19, no. 13: 8199. https://doi.org/10.3390/ijerph19138199