Degradation of Bisphenol A in an Aqueous Solution by a Photo-Fenton-Like Process Using a UV KrCl Excilamp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
3. Results
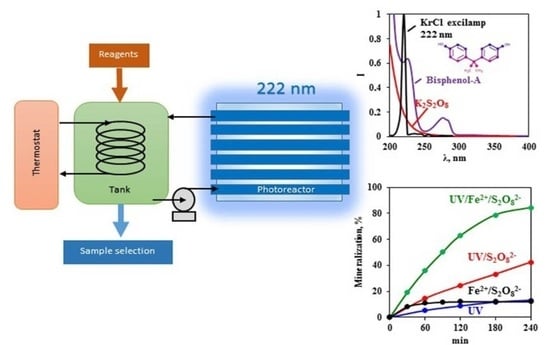
3.1. Degradation by Persulfate Activated With KrCl Excilamp (UV/S2O82−)
3.2. Degradation by a Photo-Fenton-Like Process (UV/Fe2+/S2O82−)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kim, J.-J.; Kumar, S.; Kumar, V.; Lee, Y.-M.; Kim, Y.-S.; Kumar, V. Bisphenols as a legacy pollutant, and their effects on organ vulnerability. Int. J. Environ. Res. Public Health 2019, 17, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as environmental triggers of thyroid dysfunction: Clues and evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef] [PubMed]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y.; Wang, S.; Li, W.; Niu, C.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the-art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based advanced oxidation processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Dionysiou, D.D. What is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. 2021, 189, 116627. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Ismail, L.; Ferronato, C.; Fine, L.; Jaber, F.; Chovelon, J.-M. Elimination of sulfaclozine from water with SO4•− radicals: Evaluation of different persulfate activation methods. Appl. Catal. B Environ. 2017, 573–581. [Google Scholar] [CrossRef]
- Delavaran Shiraz, A.; Takdastan, A.; Borghei, S.M. Photo-Fenton like degradation of catechol using persulfate activated by UV and ferrous ions: Influencing operational parameters and feasibility studies. J. Mol. Liq. 2018, 249, 463–469. [Google Scholar] [CrossRef]
- Graça, C.; Correia de Velosa, A.; Teixeira, A.C. Amicarbazone degradation by UVA-activated persulfate in the presence of hydrogen peroxide or Fe2+. Catal. Today 2015, 280, 80–85. [Google Scholar] [CrossRef]
- Murcia, M.D.; Vershinin, N.O.; Briantceva, N.; Gomez, M.; Gomez, E.; Cascales, E.; Hidalgo, A.M. Development of a kinetic model for the UV/H2O2 photodegradation of 2,4-dichlorophenoxiacetic acid. Chem. Eng. J. 2015, 266, 356–367. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Recent progress on application of UV excilamps for degradation of organic pollutants and microbial inactivation. Chemosphere 2012, 89, 637–647. [Google Scholar] [CrossRef]
- Gomez, M.; Murcia, M.; Gomez, J.L.; Matafonova, G.; Batoev, V.; Christofi, N. Testing a KrCl excilamp as new enhanced UV source for 4-chlorophenol degradation: Experimental results and kinetic model. Chem. Eng. Process. Process Intensif. 2010, 49, 113–119. [Google Scholar] [CrossRef]
- Sosnin, E.; Avdeev, S.; Tarasenko, V.; Skakun, V.; Schitz, D. KrCl barrier-discharge excilamps: Energy characteristics and applications (Review). Instrum. Exp. Tech. 2015, 58, 309–318. [Google Scholar] [CrossRef]
- Popova, S.; Matafonova, G.; Batoev, V. Simultaneous atrazine degradation and E. coli inactivation by UV/S2O82−/Fe2+ process under KrCl excilamp (222 nm) irradiation. Ecotoxicol. Env. Saf. 2019, 169, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, A.; Perilla, G.; Lara-Borrero, J.A.; Diez, R. KrCl and XeCl excilamps and LP-Hg lamp for UV and UV/H2O2 decolourization of dyes in water. Environ. Technol. 2020, 41, 238–250. [Google Scholar] [CrossRef]
- Sizykh, M.; Batoeva, A.; Tsydenova, O. UV-Activated persulfate oxidation of Orange III dye using KrCl excilamp. Clean Soil Air Water 2018, 46, 1700187. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Comparison of energy requirements for removal of organic micropollutants from lake water and wastewater effluents by direct UV and UV/H2O2 using excilamp. Desal. Water Treat. 2017, 85, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Gómez, M.; Murcia, M.D.; Gómez, E.; Ortega, S.; Sánchez, A.; Thaikovskaya, O.; Briantceva, N. Modelling and experimental checking of the influence of substrate concentration on the first order kinetic constant in photo-processes. J. Environ. Manag. 2016, 183, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Tchaikovskaya, O.; Karetnikova, E.; Murcia, M.; Gomez, M.; Gómez, J.L. Photodegradation of 2-methyl-4-chlorophenol in a KrCl exciplex flow-through photoreactor: A kinetic study. Desalination Water Treat. 2014, 54, 1–10. [Google Scholar] [CrossRef]
- Murcia, M.; Gomez, M.; Ortega-Requena, S.; Hidalgo, A.; Gomez, E.; Gómez, J. Degradation of congo red in a exciplex flow-through photoreactor. Kinetic study. Afinidad Barc. 2015, 72, 7–13. [Google Scholar]
- Matafonova, G.G.; Batoev, V.B. Comparison of UV and UV/H2O2 treatments using excilamps for removal of monochlorophenols in the molecular and anionic form. J. Environ. Sci. Health Part A 2012, 47, 2077–2083. [Google Scholar] [CrossRef]
- Gomez, M.; Murcia, M.D.; Gomez, J.L.; Gomez, E.; Maximo, M.F.; Garcia, A. A KrCl exciplex flow-through photoreactor for degrading 4-chlorophenol: Experimental and modelling. Appl. Catal. B Environ. 2012, 117–118, 194–203. [Google Scholar] [CrossRef]
- Murcia, M.; Gomez, M.; Gomez, E.; Gómez, J.; Christofi, N. Photodegradation of congo red using XeBr, KrCl and Cl2 barrier discharge excilamps: A kinetics study. Desalination 2011, 281, 364–371. [Google Scholar] [CrossRef]
- Canonica, S.; Meunier, L.; von Gunten, U. Phototransformation of selected pharmaceuticals during UV treatment of drinking water. Water Res. 2008, 42, 121–128. [Google Scholar] [CrossRef]
- Sorokina, E.V.; Yudina, T.P.; Bubnov, I.A.; Danilov, V.S. Assessment of iron toxicity using a luminescent bacterial test with an Escherichia coli recombinant strain. Microbiology 2013, 82, 439–444. [Google Scholar] [CrossRef]
- Sharma, J.; Mishra, I.M.; Kumar, V. Mechanistic study of photo-oxidation of Bisphenol-A (BPA) with hydrogen peroxide (H2O2) and sodium persulfate (SPS). J. Environ. Manag. 2016, 166, 12–22. [Google Scholar] [CrossRef]
- Leitner, N.K.V. Sulfate radical Ion—Based AOPs. In Advanced Oxidation Processes for Water Treatment; Stefan, M.I., Ed.; IWA Publishing: London, UK, 2018; pp. 429–460. [Google Scholar]
- Huang, W.; Bianco, A.; Brigante, M.; Mailhot, G. UVA-UVB activation of hydrogen peroxide and persulfate for advanced oxidation processes: Efficiency, mechanism and effect of various water constituents. J. Hazard. Mater. 2018, 347, 279–287. [Google Scholar] [CrossRef]
- Grčić, I.; Vujević, D.; Koprivanac, N. Modeling the mineralization and discoloration in colored systems by (US)Fe2+/H2O2/S2O82− processes: A proposed degradation pathway. Chem. Eng. J. 2010, 157, 35–44. [Google Scholar] [CrossRef]
- Kusic, H.; Peternel, I.; Ukic, S.; Koprivanac, N.; Bolanca, T.; Papic, S.; Bozic, A.L. Modeling of iron activated persulfate oxidation treating reactive azo dye in water matrix. Chem. Eng. J. 2011, 172, 109–121. [Google Scholar] [CrossRef]
- Bu, L.; Shi, Z.; Zhou, S. Modeling of Fe(II)-activated persulfate oxidation using atrazine as a target contaminant. Sep. Purif. Technol. 2016, 169, 59–65. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Hong, J.; Dong, Z.; Wang, J. Degradation of bisphenol A by persulfate activation via oxygen vacancy-rich CoFe2O4–x. Chemosphere 2019, 221, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Serra-Pérez, E.; Álvarez-Torrellas, S.; Ismael Águeda, V.; Delgado, J.A.; Ovejero, G.; García, J. Insights into the removal of Bisphenol A by catalytic wet air oxidation upon carbon nanospheres-based catalysts: Key operating parameters, degradation intermediates and reaction pathway. Appl. Surf. Sci. 2019, 473, 726–737. [Google Scholar] [CrossRef]
- Darsinou, B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sono-Activated persulfate oxidation of bisphenol A: Kinetics, pathways and the controversial role of temperature. Chem. Eng. J. 2015, 280, 623–633. [Google Scholar] [CrossRef]
- Potakis, N.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Oxidation of Bisphenol A in water by heat-activated persulfate. J. Environ. Manag. 2017, 195, 125–132. [Google Scholar] [CrossRef]






| Photo Activation System | Pollutant, Initial Concentration, mg/L | Excilamp | Conditions | Removal, % | Rate Constant | Refs. |
|---|---|---|---|---|---|---|
| UV/Fe2+/S2O82− | Atrazine, 4 | KrCl | [Fe2+] = 89.5 μM, [S2O82−] = 312 μM, | 99 | 24 × 10−2 cm2/mJ | [17] |
| Reaction time 5 min | ||||||
| UV/S2O82− | Atrazine, 4 | KrCl | [S2O82−] = 312 μM | NR * | 13 × 10−2 cm2/mJ | |
| UV/H2O2 | Methylene Blue, 18 | KrCl XeCl | C(H2O2) = 0.05% v/v | 99 30 | 1.99 min−1 0.202 min−1 | [18] |
| UV/Fe2+/S2O82− | Orange III, 10 | KrCl | [Fe2+] = 180 μM, [S2O82−] = 180 μM, Reaction time 15 min | 100 | NR | [19] |
| UV/S2O82− | Orange III, 10 | KrCl | [S2O82−] = 180 μM, Reaction time 15 min | 70 | NR | |
| UV/H2O2 | Atrazine, 1 Triclosan, 1 | KrCl | [H2O2] = 0.6 mM | NR | 3.2 × 10−2 cm2/mJ 6.2 × 10−2 cm2/mJ | [20] |
| UV/Fe2+/H2O2 | Methylene Blue, 25 | KrCl | [Fe2+] = 2 mg/L, [H2O2] = 390 μM, Reaction time 10 min | 100 | 0.12 min−1 | [21] |
| UV/H2O2 | 2,4-dichloro-phenoxiacetic acid, 50 | KrCl | [H2O2] = 452 μM, Reaction time 40 min | 100 | 0.13 min−1 | [13] |
| UV/H2O2 | 2-methyl–4-chlorophenol, 28.5 | KrCl | [H2O2] = 2 mM, Reaction time 120 min | 100 | NR | [22] |
| UV/Fe2+/H2O2 | Congo Red, 12.5 | KrCl | [Fe2+] = 3 mg/L, [H2O2] = 1.3 mM, Reaction time 90 min | 100 | 0.268 min−1 | [23] |
| UV/H2O2 | Para-chlorobenzoic acid, 25 | KrCl, XeBr | [H2O2] = 4 mM | NR | 0.13×10−2 cm2/mJ 0.10×10−2 cm2/mJ | [24] |
| UV/Fe2+/H2O2 | 4-chlorophenol, 50 | KrCl | [Fe2+] = 5 mg/L, [H2O2] = 9.7 mM, Reaction time 5 min | 100 | NR | [25] |
| UV/H2O2 | Congo Red, 100 | KrCl, XeBr Cl2 | [H2O2] = 7.2 mM, Reaction time 30 min | 100 80 50 | NR | [26] |
| [S2O82−]:[BPA], M/M | k, min−1 | τ1/2, min | R2 |
|---|---|---|---|
| - | 0.03 | 23.11 | 0.997 |
| 3:1 | 0.17 | 4.03 | 0.992 |
| 5:1 | 0.25 | 2.82 | 0.991 |
| 10:1 | 0.47 | 1.49 | 0.959 |
| 20:1 | 0.78 | 0.89 | 0.981 |
| Sample | Before | After | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 min | 30 min | ||||||||
| Bioluminescence Intensity, imp/s | T | Bioluminescence Intensity, imp/s | T | Bioluminescence Intensity, imp/s | T | ||||
| Control | Sample | Control | Sample | Control | Sample | ||||
| 1 | 21,484 | 14,582 | 30.3 | 22,586 | 19,490 | 13.7 | 22,544 | 23,905 | 0 |
| 2 | 20,233 | 16,971 | 16.1 | 22,943 | 20,666 | 9.9 | 21,104 | 24,311 | 0 |
| 3 | 19,117 | 16,275 | 14.8 | 19,217 | 18,003 | 6.3 | 20,006 | 28,033 | 0 |
| Mean | 20.4 ± 8.5 | 9.9 ± 3.7 | 0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aseev, D.; Batoeva, A.; Sizykh, M.; Olennikov, D.; Matafonova, G. Degradation of Bisphenol A in an Aqueous Solution by a Photo-Fenton-Like Process Using a UV KrCl Excilamp. Int. J. Environ. Res. Public Health 2021, 18, 1152. https://doi.org/10.3390/ijerph18031152
Aseev D, Batoeva A, Sizykh M, Olennikov D, Matafonova G. Degradation of Bisphenol A in an Aqueous Solution by a Photo-Fenton-Like Process Using a UV KrCl Excilamp. International Journal of Environmental Research and Public Health. 2021; 18(3):1152. https://doi.org/10.3390/ijerph18031152
Chicago/Turabian StyleAseev, Denis, Agniya Batoeva, Marina Sizykh, Daniil Olennikov, and Galina Matafonova. 2021. "Degradation of Bisphenol A in an Aqueous Solution by a Photo-Fenton-Like Process Using a UV KrCl Excilamp" International Journal of Environmental Research and Public Health 18, no. 3: 1152. https://doi.org/10.3390/ijerph18031152