Delayed Tibial Shaft Fracture Healing Associated with Smoking: A Systematic Review and Meta-Analysis of Observational Studies Conducted Worldwide
Abstract
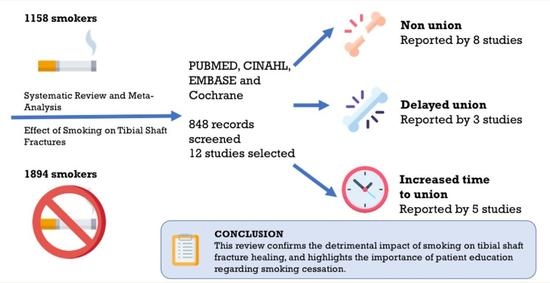
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Analysis and Summary Estimates
3. Results
3.1. Characteristics of Included Studies
3.2. Quality Assessment
3.3. Effect of Smoking on Tibial Shaft Fracture Non-Union and Healing Times
3.4. Meta-Analysis for Non-Union
3.5. Meta-Analysis for Delayed Union
3.6. Publication Bias and Meta-Regression
4. Discussion
4.1. Summary and Significance of Main Results
4.2. Study Participants
4.3. Limitations of This Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, K.E.; Ferreira, R.V. Tibial Shaft Fractures. Rev. Bras. Ortop. 2015, 46, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Court-Brown, C.M.; Caesar, B. Epidemiology of adult fractures: A review. Injury 2006, 37, 691–697. [Google Scholar] [CrossRef]
- Larsen, P.; Elsoe, R.; Hansen, S.H.; Graven-Nielsen, T.; Laessoe, U.; Rasmussen, S. Incidence and epidemiology of tibial shaft fractures. Injury 2015, 46, 746–750. [Google Scholar] [CrossRef]
- Madadi, F.; Farahmandi, M.V.; Eajazi, A.; Besheli, L.D.; Madadi, F.; Lari, M.N. Epidemiology of adult tibial shaft fractures: A 7-year study in a major referral orthopedic center in Iran. Med. Sci. Monit. 2010, 16, CR217–CR221. [Google Scholar]
- Bonafede, M.; Espindle, D.; Bower, A.G. The direct and indirect costs of long bone fractures in a working age US population. J. Med. Econ. 2013, 16, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.A.; Makaram, N.; Simpson, A.; Keating, J.F. Fracture nonunion in long bones: A literature review of risk factors and surgical management. Injury 2020, 52 (Suppl. 2), S3–S11. [Google Scholar] [CrossRef]
- Santolini, E.; West, R.; Giannoudis, P.V. Risk factors for long bone fracture non-union: A stratification approach based on the level of the existing scientific evidence. Injury 2015, 46 (Suppl. 8), S8–S19. [Google Scholar] [CrossRef]
- McMillan, T.E.; Johnstone, A.J. Technical considerations to avoid delayed and non-union. Injury 2017, 48, S64–S68. [Google Scholar] [CrossRef]
- Tian, R.; Zheng, F.; Zhao, W.; Zhang, Y.; Yuan, J.; Zhang, B.; Li, L. Prevalence and influencing factors of nonunion in patients with tibial fracture: Systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 377. [Google Scholar] [CrossRef]
- Scolaro, J.A.; Schenker, M.L.; Yannascoli, S.; Baldwin, K.; Mehta, S.; Ahn, J. Cigarette Smoking Increases Complications Following Fracture: A Systematic Review. JBJS 2014, 96, 674–681. [Google Scholar] [CrossRef]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, R.G.; Clement, R.G.E.; Edwards, K.L.; Scammell, B.E. Do smokers have greater risk of delayed and non-union after fracture, osteotomy and arthrodesis? A systematic review with meta-analysis. BMJ Open 2016, 6, e010303. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M. Reference checking for systematic reviews using Endnote. J. Med. Libr. Assoc. JMLA 2018, 106, 542. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.I.; Keating, J.F.; Court-Brown, C.M. Cigarette smoking and open tibial fractures. Injury 2001, 32, 61–65. [Google Scholar] [CrossRef]
- Moghaddam-Alvandi, A.; Zimmermann, G.; Hammer, K.; Bruckner, T.; Grützner, P.A.; von Recum, J. Cigarette smoking influences the clinical and occupational outcome of patients with tibial shaft fractures. Injury 2013, 44, 1654. [Google Scholar] [CrossRef]
- Olesen, U.K.; Juul, R.; Bonde, C.T.; Moser, C.; McNally, M.; Jensen, L.T.; Elberg, J.J.; Eckardt, H. A review of forty five open tibial fractures covered with free flaps. Analysis of complications, microbiology and prognostic factors. Int. Orthop. 2015, 39, 1159–1166. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, M.; Rothstein, H. Comprehensive Meta-Analysis; Biostat: Zagreb, Croatia, 1999. [Google Scholar]
- Alemdaroğlu, K.B.; Tiftikçi, U.; Iltar, S.; Aydoğan, N.H.; Kara, T.; Atlihan, D.; Ateşalp, A.S. Factors affecting the fracture healing in treatment of tibial shaft fractures with circular external fixator. Injury 2009, 40, 1151–1156. [Google Scholar] [CrossRef]
- Dailey, H.L.; Wu, K.A.; Wu, P.-S.; McQueen, M.M.; Court-Brown, C.M. Tibial Fracture Nonunion and Time to Healing After Reamed Intramedullary Nailing: Risk Factors Based on a Single-Center Review of 1003 Patients. J. Orthop. Trauma 2018, 32, e263–e269. [Google Scholar] [CrossRef]
- Manon, J.; Detrembleur, C.; Van de Veyver, S.; Tribak, K.; Cornu, O.; Putineanu, D. Predictors of mechanical complications after intramedullary nailing of tibial fractures. Orthop. Traumatol. Surg. Res. 2019, 105, 523–527. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Handojo, K.; Reynders, P.; Sermon, A.; Vanderschot, P.; Nijs, S. Individual risk factors for deep infection and compromised fracture healing after intramedullary nailing of tibial shaft fractures: A single centre experience of 480 patients. Injury 2015, 46, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.C.; Bosse, M.J.; MacKenzie, E.J.; Patterson, B.M.; Castillo, R.C.; Bosse, M.J.; MacKenzie, E.J.; Patterson, B.M. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J. Orthop. Trauma 2005, 19, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mundi, R.; Axelrod, D.; Heels-Ansdell, D.; Chaudhry, H.; Ayeni, O.R.; Petrisor, B.; Busse, J.W.; Thabane, L.; Bhandari, M. Nonunion in Patients with Tibial Shaft Fractures: Is Early Physical Status Associated with Fracture Healing? Cureus 2020, 12, e7649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, M.A.; Finnegan, M.; Natarajan, R.; Champine, J. Effect of Smoking on Tibial Shaft Fracture Healing. Clin. Orthop. Relat. Res. 1999, 365, 184–200. [Google Scholar] [CrossRef]
- Enninghorst, N.; McDougall, D.; Hunt, J.J.; Balogh, Z.J. Open tibia fractures: Timely debridement leaves injury severity as the only determinant of poor outcome. J. Trauma 2011, 70, 352–356. [Google Scholar] [CrossRef]
- Singh, A.; Hao, J.T.; Wei, D.T.; Liang, C.W.; Murphy, D.; Thambiah, J.; Han, C.Y. Gustilo IIIB Open Tibial Fractures: An analysis of Infection and Nonunion Rates. Indian J. Orthop. 2018, 52, 406–410. [Google Scholar] [CrossRef]
- Walker, L.M.; Preston, M.R.; Magnay, J.L.; Thomas, P.B.; El Haj, A.J. Nicotinic regulation of c-fos and osteopontin expression in human-derived osteoblast-like cells and human trabecular bone organ culture. Bone 2001, 28, 603–608. [Google Scholar] [CrossRef]
- Sloan, A.; Hussain, I.; Maqsood, M.; Eremin, O.; El-Sheemy, M. The effects of smoking on fracture healing. Surgeon 2010, 8, 111–116. [Google Scholar] [CrossRef]
- Sørensen, L.T. Wound Healing and Infection in Surgery: The Pathophysiological Impact of Smoking, Smoking Cessation, and Nicotine Replacement Therapy: A Systematic Review. Ann. Surg. 2012, 255, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Lam, D.P.; Abrishami, A.; Chan, M.T.; Chung, F. Short-term preoperative smoking cessation and postoperative complications: A systematic review and meta-analysis. Can. J. Anesth. 2012, 59, 268–279. [Google Scholar] [CrossRef] [PubMed]





| Reference | Region | Time Frame of Study | Design of Study | Follow Up | Gender | Age in Years (Mean/ST Dev or Range) | Health Outcome Definition |
|---|---|---|---|---|---|---|---|
| Adams, Keating and Court-Brown, 2001 [16] | Netherlands | 1983–1995 | Retrospective cohort | Mean 21.6 months | M (112) F (61) | (Range, 13–90); 38.7 smokers, 39.2 non-smokers | Non-union following open tibial fractures |
| Alemdaroglu et al., 2009 [21] | Turkey | 2002–2007 | Prospective cohort | Monthly, for at least 6 months | Unspecified | Mean 45.3 (range 19–75) | Time to fracture healing, delayed union, non-union |
| Castillo et al., 2005 [25] | United States of America | 1994–1997 | Prospective cohort | 3 monthly for 24 months | M (246) F (209) | Mean 33.4 (range 16–69) | Time to fracture healing |
| Dailey et al., 2018 [22] | Scotland | 1985–2007 | Retrospective cohort | Not mentioned | M (739) F (264) | Mean males = 31.3, mean females = 45.1 | Time to healing and non-union rates after reamed intramedullary nailing |
| Enninghorst et al., 2011 [28] | Australia | 2007–2009 | Prospective cohort | 12 months | M (66) F (23) | 41 ± 17 | Assessment of both non-union risk and time to union |
| Manon-et al., 2019 [23] | Belgium | 2005–2015 | Retrospective cohort | 9 months | M (105) F (66) | Mean 45.6, range: 14–95 | Time to fracture healing and delayed union rates |
| Metsemakers et al., 2015 [24] | Belgium | 2000–2012 | Retrospective cohort | Minimum 18 months, until evidence of union | M (338) F (142) | Mean 39.2, range 17–90 | Compromised fracture healing: Delayed union; non-union; requirement of secondary procedure |
| Moghaddam et al., 2011 [17] | Germany | 2002–2005 | Prospective cohort | Mean 40 months | M (61) F (24) | Mean 46, range: 18–84 at the time of injury | Time to fracture healing, delayed union, non-union |
| Mundi et al., 2020 [26] | United States, Canada, and The Netherlands | 2000–2005 | Prospective cohort | 12 months | M (709) F (231) | 40.9 ± 15.6 | Non-union rates |
| Olesen et al., 2015 [18] | Denmark | 2002–2013 | Retrospective cohort | 12 months | M (32) F (13) | 42 ± 18, range 16–71 | Non-union rates |
| Schmitz et al., 1999 [27] | USA | 1990–1993 | Prospective cohort | 12 months | M (73) F (30) | Smoker: 35.6 ± 1.7, Non-smoker: 35.8 ± SD 1.9 | Time to clinical union, non-union, time to radiographic healing |
| Singh et al., 2018 [29] | Singapore | 2000–2013 | Retrospective cohort | Minimum 6 months, until union | M (111) F (8) | 38.2, range 18–70 | Time to fracture union and non-union |
| Reference | Exposure to Smoking | Non-Exposed to Smoking | Smoking Habit by Gender | Definition of Smoking | Adjusted Odds Ratio and 95% CI and p Value | Healing Time Smokers in Weeks, Mean ± SD | Healing Time Non-Smokers, Mean ± SD in Weeks |
|---|---|---|---|---|---|---|---|
| Adams, Keating and Court-Brown, 2001 [16] | 140 smokers | 133 | 12 males, 28 females smokers; 100 males, 33 females non-smokers | 10 or more cigarettes per day, not intermittently | OR 1.48 (95% CI 0.87 to 2.51) non-union, p = 0.14 | 32.3 | 27.8 |
| Alemdaroglu et al., 2009 [21] | 13 smokers | 19 | Not mentioned | Not defined | p = 0.158 | 27.54 ± 11.609 | 21.37 ± 5.079 |
| Castillo et al., 2005 [25] | 82 previous smoker, 105 current smoker | 81 | 73% males, 27% females | Never smoked, previous smoker (100 or more cigarettes over the course of his or her lifetime), current smoker | Current smokers versus non-smokers (p = 0.01), whereas previous smokers versus non-smokers (p = 0.04) | 47.8 previous smoker, 42.9 current smoker | 40.1 |
| Dailey et al., 2018 [22] | 244 smokers | 261 | 739 males, 264 females | Patients with records for smoking | Smoker OR: 1.15; 95% CI: 0.70–1.89, p = 0.572 for non-union rate, p = 0.006 for time to union | 18 | 18 |
| Enninghorst et al., 2011 [28] | 31 smokers | 90 | 74% (66) male, 26% females | Not mentioned | Non-union: OR: 2.26; 95% CI: 0.83–6.15 | Not mentioned | Not mentioned |
| Manon-et al., 2019 [23] | 40 smokers | 131 | 105 males and 66 females | Not defined | Delayed union: OR: 6.06; 95% CI: 1.02–36.16, p = 0.048 | Not mentioned | Not mentioned |
| Metsemakers et al., 2015 [24] | 146 smokers | 334 | 338 male patients (70.4%) and 142 female patients (29.6%) | Active smokers at time of the initial procedure | Delayed union: OR: 1.74; 95% CI: 0.87–3.49, p = 0.120; non-union: OR: 0.96; CI 0.48–1.95, p = 0.915 | Not mentioned | Not mentioned |
| Moghaddam et al., 2011 [17] | 46 smokers | 39 | 61 men (72%) and 24 women (28%) | Self-reported smoking status | Delayed union OR: 2.92; 95% CI: 0.73 to 11.65; Non-union OR: 20.01, 95% CI: 1.125 to 356.08 p = 0.0007 | 17.4 | 11.9 |
| Mundi et al., 2020 [26] | 299 smokers | 640 | 709 males, 231 females | Not defined | Non-union: OR: 1.39; CI: 0.92–2.10, p = 0.113 | Not mentioned | Not measured |
| Olesen et al., 2015 [18] | 15 smokers | 30 | 13 women and 32 men | Data from patient records regarding tobacco use | Non-union: OR: 3.89, 95% CI: 1.08–13.96, p ≤ 0.058 | Not mentioned | Not measured |
| Schmitz et al., 1999 [27] | 76 smokers | 59 | 73 (31 smoker, 13 non-smoker) males, 30 (13 smoker, 17 non-smoker) females | Smoke more than 5 cigarettes per day at the time of fracture | Not mentioned | Not mentioned | Not mentioned |
| Singh et al., 2018 [29] | 26 smokers | 77 | 111 males (93.2%) and eight females | Not defined | Not mentioned | Revision (due to non-union)/delayed union in smokers versus smokers p = 0.0381 |
| Reference | Overall Quality Assessment-Max 9 | Selection | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Was Not Present at Start of Study | Assessment | Duration of Follow Up | Adequacy of Follow Up (>80%) | |||
| Adams, Keating and Court-Brown, 2001 [16] | 7 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Alemdaroglu et al., 2009 [21] | 5 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Castillo et al., 2005 [25] | 9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Dailey et al., 2018 [22] | 8 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 |
| Enninghorst et al., 2011 [28] | 9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Manon-et al., 2019 [23] | 8 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | Unspecified- retrospective study |
| Metsemakers et al., 2015 [24] | 9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Moghaddam et al., 2011 [17] | 8 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Mundi et al., 2020 [26] | 9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Olesen et al., 2015 [18] | 6 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | Unspecified- retrospective study |
| Schmitz et al., 1999 [27] | 9 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Singh et al., 2018 [29] | 7 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahajan, A.; Kumar, N.; Gupta, B. Delayed Tibial Shaft Fracture Healing Associated with Smoking: A Systematic Review and Meta-Analysis of Observational Studies Conducted Worldwide. Int. J. Environ. Res. Public Health 2021, 18, 10228. https://doi.org/10.3390/ijerph181910228
Mahajan A, Kumar N, Gupta B. Delayed Tibial Shaft Fracture Healing Associated with Smoking: A Systematic Review and Meta-Analysis of Observational Studies Conducted Worldwide. International Journal of Environmental Research and Public Health. 2021; 18(19):10228. https://doi.org/10.3390/ijerph181910228
Chicago/Turabian StyleMahajan, Akanksha, Narinder Kumar, and Bhawna Gupta. 2021. "Delayed Tibial Shaft Fracture Healing Associated with Smoking: A Systematic Review and Meta-Analysis of Observational Studies Conducted Worldwide" International Journal of Environmental Research and Public Health 18, no. 19: 10228. https://doi.org/10.3390/ijerph181910228