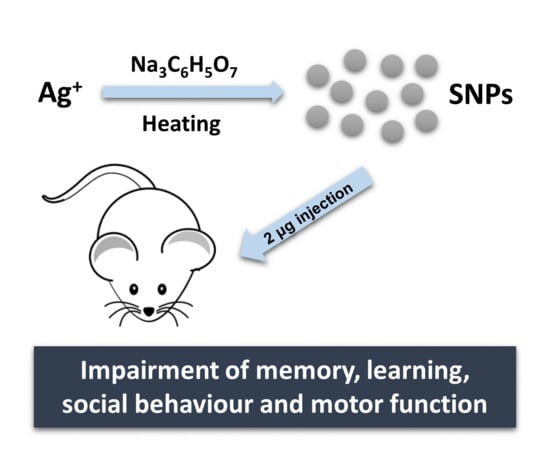
The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design, Synthesis and Characterization of AgNPs
2.2. In Vivo Studies
2.2.1. Morris Water Maze Test (MWM)
2.2.2. Three-Chambers Social Apparatus Test
2.2.3. Rotarod Performance Test
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matteucci, F.; Giannantonio, R.; Calabi, F.; Agostiano, A.; Gigli, G.; Rossi, M. Deployment and Exploitation of Nanotechnology Nanomaterials and Nanomedicine. In AIP Conference Proceedings, 2018; AIP Publishing: Melville, NY, USA, 2018; p. 020001. [Google Scholar]
- Global Market Insights, Inc. Glycerol Market Size by Application (Personal Care, Polyether Polyol Applications, Alkyd Resins), by Source (Biodiesel, Fatty Alcohols, Fatty Acids), Industry Analysis Report, Regional Outlook, Price Trend, Downstream Potential, Competitive Market Share & Forecast, 2012–2022; Global Market Insights, Inc.: Selbyville, DE, USA, 2016. [Google Scholar]
- Zivic, F.; Grujovic, N.; Mitrovic, S.; Ahad, I.U.; Brabazon, D. Characteristics and Applications of Silver Nanoparticles. In Commercialization of Nanotechnologies–A Case Study Approach; Springer: Basel, Switzerland, 2018; pp. 227–273. [Google Scholar]
- Asharani, P.; Gong, Z.; Hande, M.P.; Valiyaveettil, S. Potential Health Impacts of Silver Nanoparticles; Chemical Research in Toxicology: Washington, DC, USA, 2007; p. 2009. [Google Scholar]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.; Riediker, M. Use of nanoparticles in Swiss industry: A targeted survey. Environ. Sci. Technol. 2008, 42, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Breitner, E.K.; Burns, K.E.; Hussain, S.M.; Comfort, K.K. Implementation of physiological fluids to provide insight into the characterization, fate, and biological interactions of silver nanoparticles. Nanotechnology 2018, 29, 254001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, L.S.; Laurencin, C.T. Silver nanoparticles: Synthesis and therapeutic applications. J. Biomed. Nanotechnol. 2007, 3, 301–316. [Google Scholar] [CrossRef]
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol. Brain 2013, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Vlasceanu, G.M.; Tiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M. Applications and Toxicity of Silver Nanoparticles: A Recent Review. Curr. Top. Med. Chem. 2015, 15, 1596–1604. [Google Scholar] [CrossRef]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Mechanisms of Toxicity of Ag Nanoparticles in Comparison to Bulk and Ionic Ag on Mussel Hemocytes and Gill Cells. PLoS ONE 2015, 10, e0129039. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles-Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef]
- Vidmar, J.; Loeschner, K.; Correia, M.; Larsen, E.H.; Manser, P.; Wichser, A.; Boodhia, K.; Al-Ahmady, Z.S.; Ruiz, J.; Astruc, D.; et al. Translocation of silver nanoparticles in the ex vivo human placenta perfusion model characterized by single particle ICP-MS. Nanoscale 2018, 10, 11980–11991. [Google Scholar] [CrossRef]
- Tang, J.; Xiong, L.; Zhou, G.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Lu, S.; Wan, Z.; et al. Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. J. Nanosci. Nanotechnol. 2010, 10, 6313–6317. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Carter, D.A.; Leo, B.F.; Ruenraroengsak, P.; Chen, S.; Goode, A.E.; Theodorou, I.G.; Chung, K.F.; Carzaniga, R.; Shaffer, M.S.P.; Dexter, D.T.; et al. Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Sci. Rep. 2017, 7, 42871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arieff, A.I. Aluminum and the pathogenesis of dialysis encephalopathy. Am. J. Kidney Dis. 1985, 6, 317–321. [Google Scholar] [CrossRef]
- Parkinson, I.; Ward, M.; Kerr, D. Dialysis encephalopathy, bone disease and anaemia: The aluminum intoxication syndrome during regular haemodialysis. J. Clin. Pathol. 1981, 34, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Elliott, H.; Boddy, K.; Haywood, J.; Henderson, I.; Harvey, I.; Kennedy, A. Whole body aluminium in chronic renal failure and dialysis encephalopathy. Clin. Nephrol. 1980, 14, 198–200. [Google Scholar] [PubMed]
- Pascoe, M.; Gregory, M. Dialysis Encephalopathy-Aluminum Concentration in Dialysate and Brain; Kidney International, 1979; Blackwell Science Inc.: Malden, MA, USA, 1979; p. 90. [Google Scholar]
- Rodella, L.; Ricci, F.; Borsani, E.; Stacchiotti, A.; Foglio, E.; Favero, G.; Rezzani, R.; Mariani, C.; Bianchi, R. Aluminium exposure induces Alzheimer′s disease-like histopathological alterations in mouse brain. Histol. Histopathol. 2008, 23, 433–440. [Google Scholar] [PubMed]
- McLachlan, D.; Fraser, P.; Dalton, A. Aluminum and the pathogenesis of Alzheimer′s disease: A summary of evidence. CIBA Found. Symp. 1992, 169, 87–108. [Google Scholar]
- Good, P.; Perl, P. Laser Microprobe Mass Analysis (lamma) Evidence of Aluminum Accumulation In The Granules of Granulovacuolar Degeneration (gvd) of Alzheimer′s Disease (ad) And Guam Als/parkinsonism Dementia Complex (als-pdc). J. Neuropathol. Exper. Neurol. 1990, 49, 317. [Google Scholar] [CrossRef]
- Garruto, R.M.; Fukatsu, R.; Yanagihara, R.; Gajdusek, D.C.; Hook, G.; Fiori, C.E. Imaging of calcium and aluminum in neurofibrillary tangle-bearing neurons in parkinsonism-dementia of Guam. Proc. Nat. Acad. Sci. USA 1984, 81, 1875–1879. [Google Scholar] [CrossRef]
- Perl, D.P.; Gajdusek, D.C.; Garruto, R.M.; Yanagihara, R.T.; Gibbs, C.J. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and Parkinsonism-dementia of Guam. Science 1982, 217, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Ali, S.F.; Hussain, S.M.; Schlager, J.J.; Sharma, A. Influence of engineered nanoparticles from metals on the blood-brain barrier permeability, cerebral blood flow, brain edema and neurotoxicity. An experimental study in the rat and mice using biochemical and morphological approaches. J. Nanosci. Nanotechnol. 2009, 9, 5055–5072. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Wen, H.; Shao, A.; Xu, L. Silver Nanoparticle Exposure Induces Neurotoxicity in the Rat Hippocampus without Increasing the Blood-Brain Barrier Permeability. J. Biomed. Nanotechnol. 2018, 14, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Trickler, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W., Jr.; et al. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol. Sci. 2010, 118, 160–170. [Google Scholar] [CrossRef]
- Danila, O.O.; Berghian, A.S.; Dionisie, V.; Gheban, D.; Olteanu, D.; Tabaran, F.; Baldea, I.; Katona, G.; Moldovan, B.; Clichici, S.; et al. The effects of silver nanoparticles on behavior, apoptosis and nitro-oxidative stress in offspring Wistar rats. Nanomedicine-UK 2017, 12, 1455–1473. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Yu, C.H.; Tan, Y.; Hou, Z.; Li, M.; Shao, F.; Lu, X.X. Effects of prenatal exposure to silver nanoparticles on spatial cognition and hippocampal neurodevelopment in rats. Environ. Res. 2015, 138, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wesierska, M.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Dudek, J.; Polkowska-Motrenko, H.; Audinot, J.N.; Gutleb, A.C.; Lankoff, A.; Kruszewski, M. Silver ions are responsible for memory impairment induced by oral administration of silver nanoparticles. Toxicol. Lett. 2018, 290, 133–144. [Google Scholar] [CrossRef]
- Antsiferova, A.; Kopaeva, M.; Kashkarov, P. Effects of Prolonged Silver Nanoparticle Exposure on the Contextual Cognition and Behavior of Mammals. Materials 2018, 11, 558. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Greish, K.; Mathur, A.; Bakhiet, M.; Taurin, S. Nanomedicine: Is it lost in translation? Ther. Deliv. 2018, 9, 269–285. [Google Scholar] [CrossRef]
- Tang, J.; Xiong, L.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Xi, T. Distribution, translocation and accumulation of silver nanoparticles in rats. J. Nanosci. Nanotechnol. 2009, 9, 4924–4932. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Hutchison, G.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010, 40, 328–346. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greish, K.; Alqahtani, A.A.; Alotaibi, A.F.; Abdulla, A.M.; Bukelly, A.T.; Alsobyani, F.M.; Alharbi, G.H.; Alkiyumi, I.S.; Aldawish, M.M.; Alshahrani, T.F.; et al. The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice. Int. J. Environ. Res. Public Health 2019, 16, 148. https://doi.org/10.3390/ijerph16010148
Greish K, Alqahtani AA, Alotaibi AF, Abdulla AM, Bukelly AT, Alsobyani FM, Alharbi GH, Alkiyumi IS, Aldawish MM, Alshahrani TF, et al. The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice. International Journal of Environmental Research and Public Health. 2019; 16(1):148. https://doi.org/10.3390/ijerph16010148
Chicago/Turabian StyleGreish, Khaled, Abdulelah Abdullah Alqahtani, Abdulla Falah Alotaibi, Ahmed Mohamed Abdulla, Aysha Tariq Bukelly, Fanar Mohammed Alsobyani, Ghazi Hamad Alharbi, Israa Saeed Alkiyumi, Majed Mutlaq Aldawish, Turki Fahad Alshahrani, and et al. 2019. "The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice" International Journal of Environmental Research and Public Health 16, no. 1: 148. https://doi.org/10.3390/ijerph16010148