Structural Elucidation of Glycosaminoglycans in the Tissue of Flounder and Isolation of Chondroitin Sulfate C
Abstract
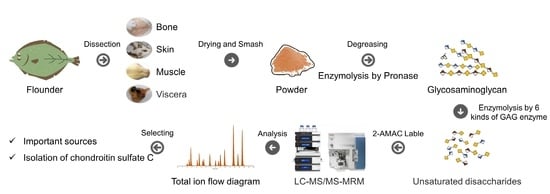
:1. Introduction
2. Results and Discussion
2.1. Data Processing
2.2. Content and Disaccharide Composition of CS in Different Flounder Tissues
2.3. Content and Disaccharide Composition of HS in Different Flounder Tissues
2.4. Content of HA in Different Flounder Tissues
2.5. The Sulfation Degree of CS and HS in Flounder Tissue
2.6. Structural Analysis of CS from Fish Bone
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Sample Preparation and Labeling
3.3. HPLC-MS/MS Analysis
3.4. Extraction and Purification of CS from Flounder Bone
3.5. Molecular Weight Determination by HPGPC-MALLS
3.6. Structural Analysis of CS by NMR and HPLC-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Veterinární Medicína 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Mestechkina, N.M.; Shcherbukhin, V.D. Sulfated polysaccharides and their anticoagulant activity: A review. Appl. Biochem. Microbiol. 2010, 46, 267–273. [Google Scholar] [CrossRef]
- Maaroufi, R.M.; Giordano, P.; Triadou, P.; Tapon-Bretaudière, J.; Dautzenberg, M.; Fischer, A. Effect of oversulfated dermatan sulfate derivatives on platelet aggregation. Thromb. Res. 2007, 120, 615–621. [Google Scholar] [CrossRef]
- Pichert, A.; Samsonov, S.A.; Theisgen, S.; Thomas, L.; Baumann, L.; Schiller, J.; Beck-Sickinger, A.G.; Huster, D.; Pisabarro, M.T. Characterization of the interaction of interleukin-8 with hyaluronan, chondroitin sulfate, dermatan sulfate and their sulfated derivatives by spectroscopy and molecular modeling. Glycobiology 2012, 22, 134–145. [Google Scholar] [CrossRef]
- Ikeda, Y.; Charef, S.; Ouidja, M.; Barbier-Chassefière, V.; Sineriz, F.; Duchesnay, A.; Narasimprakash, H.; Martelly, I.; Kern, P.; Barritault, D.; et al. Synthesis and biological activities of a library of glycosaminoglycans mimetic oligosaccharides. Biomaterials 2011, 32, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Pomin, V. The sea as a rich source of structurally unique glycosaminoglycans and mimetics. Microorganisms 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.S.; Cavalcante, R.S.; Palhares, L.C.G.F.; Hughes, A.J.; Andrade, G.P.V.; Yates, E.A.; Nader, H.B.; Lima, M.A.; Chavante, S.F. A non-hemorrhagic hybrid heparin/heparan sulfate with anticoagulant potential. Carbohydr. Polym. 2014, 99, 372–378. [Google Scholar] [CrossRef]
- Tamura, J.; Arima, K.; Imazu, A.; Tsutsumishita, N.; Fujita, H.; Yamane, M.; Matsumi, Y. Sulfation patterns and the amounts of chondroitin sulfate in the diamond squid, thysanoteuthis rhombus. Biosci. Biotechnol. Biochem. 2014, 73, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xue, C.; Yin, L.A.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Silchenko, A.S.; Grebnev, B.B.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Fucosylated chondroitin sulfates from the sea cucumbers paracaudina chilensis and holothuria hilla: Structures and anticoagulant activity. Mar. Drugs 2020, 18, 540. [Google Scholar] [CrossRef] [PubMed]
- Maccari, F.; Ferrarini, F.; Volpi, N. Structural characterization of chondroitin sulfate from sturgeon bone. Carbohydr. Res. 2010, 345, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Song, J.; Zhang, L.; Wang, S.; Wu, R.; Ma, C.; Li, P. Chemical characteristics and antithrombotic effect of chondroitin sulfates from sturgeon skull and sturgeon backbone. Carbohydr. Polym. 2015, 123, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Krichen, F.; Karoud, W.; Sila, A.; Abdelmalek, B.E.; Ghorbel, R.; Ellouz-Chaabouni, S.; Bougatef, A. Extraction, characterization and antimicrobial activity of sulfated polysaccharides from fish skins. Int. J. Biol. Macromol. 2015, 75, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Krichen, F.; Volpi, N.; Sila, A.; Maccari, F.; Mantovani, V.; Galeotti, F.; Ellouz-Chaabouni, S.; Bougatef, A. Purification, structural characterization and antiproliferative properties of chondroitin sulfate/dermatan sulfate from tunisian fish skins. Int. J. Biol. Macromol. 2017, 95, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, M.; Dhahri, M.; Bertholon, I.; Ollivier, V.; Bataille, I.; Ajzenberg, N.; Hassine, M.; Jandrot-Perrus, M.; Chaubet, F.; Maaroufi, R.M. Characterization of a novel dermatan sulfate with high antithrombin activity from ray skin (raja radula). Thromb. Res. 2009, 123, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Garnjanagoonchorn, W.; Wongekalak, L.; Engkagul, A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. Process Intensif. 2007, 46, 465–471. [Google Scholar] [CrossRef]
- Brewer, C.F.; Keiser, H. Carbon-13 nuclear magnetic resonance study of chondroitin 4-sulfate in the proteoglycan of bovine nasal cartilage. Proc. Natl. Acad. Sci. USA 1975, 72, 3421–3423. [Google Scholar] [CrossRef]
- Mucci, A.; Schenetti, L.; Volpi, N. 1h and 13c nuclear magnetic resonance identification and characterization of components of chondroitin sulfates of various origin. Carbohydr. Polym. 2000, 41, 37–45. [Google Scholar] [CrossRef]
- Maccari, F.; Galeotti, F.; Volpi, N. Isolation and structural characterization of chondroitin sulfate from bony fishes. Carbohydr. Polym. 2015, 129, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, M.; Naggi, A.; Guglieri, S.; Santarsiero, R.; Torri, G. Complex glycosaminoglycans: Profiling substitution patterns by two-dimensional nuclear magnetic resonance spectroscopy. Anal. Biochem. 2005, 337, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Shrikanth, C.B.; Sanjana, J.; Chilkunda, N.D. One-pot analysis of sulfated glycosaminoglycans. Glycoconj. J. 2018, 35, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Chen, M.; Zhang, Z.; Corte, A.D.; Souza, C.; Giugliani, R.; Pan, L.; Qiu, Y.; Amaravadi, L.; Wu, J. A novel LC-MS/MS assay to quantify dermatan sulfate in cerebrospinal fluid as a biomarker for mucopolysaccharidosis ii. Bioanalysis 2018, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, L.; Overdier, K.H.; Ammons, L.A.; Douglas, I.S.; Burlew, C.C.; Zhang, F.; Schmidt, E.P.; Chi, L.; Linhardt, R.J. Analysis of total human urinary glycosaminoglycan disaccharides by liquid chromatography–tandem mass spectrometry. Anal. Chem. 2015, 87, 6220–6227. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, L.; Tian, F.; Zhang, L.; Xue, C.; Linhardt, R.J. Glycosaminoglycanomics of cultured cells using a rapid and sensitive lc-ms/ms approach. Acs Chem. Biol. 2015, 10, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lang, Y.; Li, Q.; Jin, X.; Li, G.; Yu, G. Glycosaminoglycanomic profiling of human milk in different stages of lactation by liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 258, 231–236. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.D.R. Hyaluronic acid and chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, P.; Liu, B.; Ai, C.; Zhou, D.; Song, S.; Zhu, B. Identification and quantification of uronic acid-containing polysaccharides in tissues of russian sturgeon (acipenser gueldenstaedtii) by HPLC-MS/MS and HPLC-MSN. Eur. Food Res. Technol. 2017, 243, 1201–1209. [Google Scholar] [CrossRef]
- Bougatef, H.; Krichen, F.; Capitani, F.; Amor, I.B.; Maccari, F.; Mantovani, V.; Galeotti, F.; Volpi, N.; Bougatef, A.; Sila, A. Chondroitin sulfate/dermatan sulfate from corb (sciaena umbra) skin: Purification, structural analysis and anticoagulant effect. Carbohydr. Polym. 2018, 196, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Fujita, H.; Toita, R.; Imazu-Okada, A.; Tsutsumishita-Nakai, N.; Takeda, N.; Nakao, Y.; Wang, H.; Kawano, M.; Matsushita, K.; et al. Amounts and compositional analysis of glycosaminoglycans in the tissue of fish. Carbohydr. Res. 2013, 366, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.V.; Rossi, G.R.; Iacomini, M.; Sassaki, G.L.; Trindade, E.S.; Cipriani, T.R. Viscera of fishes as raw material for extraction of glycosaminoglycans of pharmacological interest. Int. J. Biol. Macromol. 2019, 121, 239–248. [Google Scholar] [CrossRef] [PubMed]
- López-Senra, E.; Casal-Beiroa, P.; López-álvarez, M.; Serra, J.; González, P.; Valcarcel, J.; Vázquez, J.A.; Burguera, E.F.; Blanco, F.J.; Magalhães, J. Impact of prevalence ratios of chondroitin sulfate (cs)- 4 and -6 isomers derived from marine sources in cell proliferation and chondrogenic differentiation processes. Mar. Drugs 2020, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wei, M.; Fang, J.; Dai, W.; Sun, T.; Liu, Q.; Gong, G.; Liu, Y.; Song, S.; Ma, F.; et al. Preparation of chondroitin sulfates with different molecular weights from bovine nasal cartilage and their antioxidant activities. Int. J. Biol. Macromol. 2020, 152, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Novoa-Carballal, R.; Pérez-Martín, R.; Blanco, M.; Sotelo, C.G.; Fassini, D.; Nunes, C.; Coimbra, M.A.; Silva, T.H.; Reis, R.L.; Vázquez, J.A. By-products of scyliorhinus canicula, prionace glauca and raja clavata: A valuable source of predominantly 6s sulfated chondroitin sulfate. Carbohydr. Polym. 2017, 157, 31–37. [Google Scholar] [CrossRef]
- Meng, D.; Leng, X.; Zhang, Y.; Luo, J.; Du, H.; Takagi, Y.; Dai, Z.; Wei, Q. Comparation of the structural characteristics and biological activities of chondroitin sulfates extracted from notochord and backbone of Chinese sturgeon (acipenser sinensis). Carbohydr. Res. 2022, 522, 108685. [Google Scholar] [CrossRef]






| Sample | SM | P | LF | G | PB | PC | CH | |
|---|---|---|---|---|---|---|---|---|
| CS-S | bone | 0.80 | 0.88 | 0.89 | 0.84 | 0.85 | 0.80 | 0.87 |
| muscle | 1.05 | 1.05 | 0.90 | 0.78 | 0.91 | 0.82 | 0.86 | |
| skin | 0.74 | 0.81 | 1.02 | 0.71 | 0.79 | 0.64 | 0.90 | |
| viscera | 0.98 | 1.07 | 0.96 | - | 0.93 | 0.91 | 0.86 | |
| HS-S | bone | 0.60 | 0.34 | 0.73 | 0.72 | 0.40 | 0.23 | 0.34 |
| muscle | 0.60 | 0.95 | 0.99 | 0.86 | 0.74 | 0.87 | 0.85 | |
| skin | 0.56 | 0.78 | 0.79 | 0.67 | 0.63 | 0.71 | 0.71 | |
| viscera | 0.71 | 0.87 | 0.75 | - | 0.71 | 1.03 | 0.77 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, W.; Gong, H.; Jiang, Y.; Liu, R.; Yu, G.; Li, G.; Cai, C. Structural Elucidation of Glycosaminoglycans in the Tissue of Flounder and Isolation of Chondroitin Sulfate C. Mar. Drugs 2024, 22, 198. https://doi.org/10.3390/md22050198
Wang Z, Wang W, Gong H, Jiang Y, Liu R, Yu G, Li G, Cai C. Structural Elucidation of Glycosaminoglycans in the Tissue of Flounder and Isolation of Chondroitin Sulfate C. Marine Drugs. 2024; 22(5):198. https://doi.org/10.3390/md22050198
Chicago/Turabian StyleWang, Zhe, Weiwen Wang, Hao Gong, Yudi Jiang, Renjie Liu, Guangli Yu, Guoyun Li, and Chao Cai. 2024. "Structural Elucidation of Glycosaminoglycans in the Tissue of Flounder and Isolation of Chondroitin Sulfate C" Marine Drugs 22, no. 5: 198. https://doi.org/10.3390/md22050198