Utilization of Lumpfish (Cyclopterus lumpus) Skin as a Source for Gelatine Extraction Using Acid Hydrolysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield and Proximate Composition of Lumpfish Carcass
2.2. Amino Acid Profile and Hydroxyproline Content of Lumpfish Skin
2.3. Total Extraction Yield
2.4. Total Amino Acid Composition of Isolated Gelatine
2.5. Total Protein and Gelatine Content in the Extracted Gelatine Samples
2.6. Molecular-Weight Distribution
2.7. Dry Matter and Ash Content
2.8. Rheological Properties
3. Materials and Methods
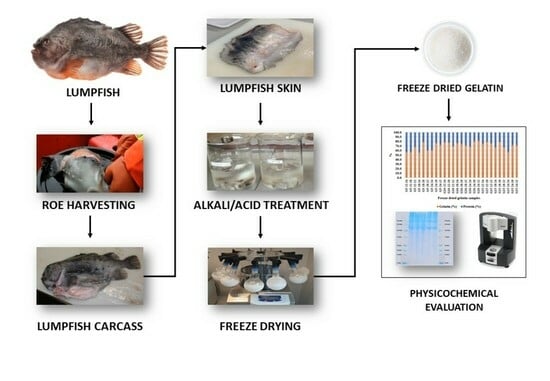
3.1. Preparation of Raw Material
3.2. Extraction of Gelatine
3.2.1. Pre-Treatment Process
3.2.2. Acid Extraction
3.2.3. Freeze-Drying
3.3. Analysis
3.3.1. Proximate Composition and Total Amino Acid Composition of the Lumpfish Fractions
3.3.2. Extraction Yield, Total Amino Acid Composition, and Hydroxyproline and Gelatine Content Analyses of the Freeze-Dried Gelatine Samples
3.3.3. Protein Analysis
3.3.4. Molecular-Weight Distribution
3.4. Rheological Properties
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Powell, A.; Treasurer, J.W.; Pooley, C.L.; Keay, A.J.; Lloyd, R.; Imsland, A.K.; de Leaniz, C.G. Use of lumpfish for sea-lice control in salmon farming: Challenges and opportunities. Rev. Aquac. 2018, 10, 683–702. [Google Scholar] [CrossRef]
- Kennedy, J.; Durif, C.M.F.; Florin, A.-B.; Fréchet, A.; Gauthier, J.; Hüssy, K.; Jónsson, S.; Ólafsson, H.G.; Post, S.; Hedeholm, R.B. A brief history of lumpfishing, assessment, and management across the North Atlantic. ICES J. Mar. Sci. 2018, 76, 181–191. [Google Scholar] [CrossRef]
- Ageeva, T.N.; Lorentzen, G.; Nilsen, H.A.; Lian, K. Lumpfish (Cyclopterus Lumpus) Used as Cleaner Fish: Characterization and Suitability for Human Consumption. Appl. Food Res. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Thong, N.T.; Ngoc, Q.T.K.; Voldnes, G. Consumer’s perception and acceptance of lumpfish used in salmon cages. Aquac. Int. 2023. [Google Scholar] [CrossRef]
- Brooker, A.J.; Papadopoulou, A.; Gutierrez, C.; Rey, S.; Davie, A.; Migaud, H. Sustainable Production and Use of Cleaner Fish for the Biological Control of Sea Lice: Recent Advances and Current Challenges. Vet. Rec. 2018, 183, 383. [Google Scholar] [CrossRef] [PubMed]
- European Union. Scientific, Technical and Economic Committee for Fisheries (Stecf)—Landing Obligation in Eu Fisheries (Stecf-13-23); Doerner, H., Graham, N., Eds.; European Union: Luxembourg, 2013; p. 115. [Google Scholar]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine Collagen and Its Derivatives: Versatile and Sustainable Bio-Resources for Healthcare. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110963. [Google Scholar] [CrossRef] [PubMed]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen Derived from Fish Industry Waste: Progresses and Challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef]
- Raman, M.; Gopakumar, K. Fish Collagen and Its Applications in Food and Pharmaceutical Industry: A Review. EC Nutr. 2018, 13, 752–767. [Google Scholar]
- Oliveira, V.d.M.; Assis, C.R.D.; Costa, B.d.A.M.; Neri, R.C.d.A.; Monte, F.T.D.; Freitas, H.M.S.d.C.V.; França, R.C.P.; Santos, J.F.; Bezerra, R.d.S.; Porto, A.L.F. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kim, M.J. Fish by-Product Collagen Extraction Using Different Methods and Their Application. Mar. Drugs 2024, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Basby, M.; Jeppesen, V.F.; Huss, H.H. Chemical Composition of Fresh and Salted Lumpfish (Cyclopterus Lumpus) Roe. J. Aquat. Food Prod. Technol. 1998, 7, 7–21. [Google Scholar] [CrossRef]
- Davenport, J.; Kjørsvik, E. Buoyancy in the Lumpsucker Cyclopterus Lumpus. J. Mar. Biol. Assoc. 1986, 66, 159–174. [Google Scholar] [CrossRef]
- Bechtel, P.J.; Bland, J.M.; Bett-Garber, K.L.; Grimm, C.C.; Brashear, S.S.; Lloyd, S.W.; Watson, M.A.; Lea, J.M. Chemical and Nutritional Properties of Channel and Hybrid Catfish Byproducts. Food Sci. Nutr. 2017, 5, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Zhou, J.; Barba, F.J.; Lorenzo, J.M. Nutritional Characterization of Sea Bass Processing by-Products. Biomolecules 2020, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Boran, G.; Regenstein, J.M. Chapter 5—Fish Gelatin. In Advances in Food and Nutrition Research; Steve, L.T., Ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 119–143. [Google Scholar]
- Fajar, A.; Warsinggih; Syarifuddin, E.; Hendarto, J.; Labeda, I.; Lusikooy, R.E.; Mappincara; Dani, M.I.; Sampetoding, S.; Kusuma, M.I.; et al. The relationship between glycine levels in collagen in the anterior rectus sheath tissue and the onset of indirect inguinal hernia: A cross-sectional study. Ann. Med. Surg. 2022, 73, 103166. [Google Scholar]
- Stoilov, I.; Starcher, B.C.; Mecham, R.P.; Broekelmann, T.J. Chapter 7—Measurement of Elastin, Collagen, and Total Protein Levels in Tissues. In Methods in Cell Biology; Robert, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 133–146. [Google Scholar]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Nalinanon, S.; Shahidi, F. Fish Collagen. In Food Biochemistry and Food Processing; Wiley: Hoboken, NJ, USA, 2012; pp. 365–387. [Google Scholar]
- Fan, H.Y.; Dumont, M.-J.; Simpson, B.K. Preparation and Physicochemical Characterization of Films Prepared with Salmon Skin Gelatin Extracted by a Trypsin-Aided Process. Curr. Res. Food Sci. 2020, 3, 146–157. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction and Characterisation of Gelatine from Atlantic Salmon (Salmo Salar) Skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef]
- Luo, Y.; Matejic, T.; Ng, C.-K.; Nunnally, B.; Porter, T.; Raso, S.; Rouse, J.; Shang, T.; Steckert, J. 8—Characterization and Analysis of Biopharmaceutical Proteins. In Separation Science and Technology; Satinder, A., Stephen, S., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 283–359. [Google Scholar]
- Islam, R.; Yuhi, T.; Meng, D.; Yoshioka, T.; Ogata, Y.; Ura, K.; Takagi, Y. Purity and Properties of Gelatins Extracted from the Head Tissue of the Hybrid Kalamtra Sturgeon. LWT 2021, 142, 110944. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I. 6—Gelatin. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 142–163. [Google Scholar]
- Kołodziejska, I.; Skierka, E.; Sadowska, M.; Kołodziejski, W.; Niecikowska, C. Effect of Extracting Time and Temperature on Yield of Gelatin from Different Fish Offal. Food Chem. 2008, 107, 700–706. [Google Scholar] [CrossRef]
- Gerry, R.; Sirinupongb, N.; Samakradhamrongthaia, R.S. Effect of Extraction Ph and Temperature on Yield and Physicochemicalproperties of Gelatin from Atlantic Salmon (Salmo Salar) Skin. Agric. Nat. Resour. 2022, 56, 687–696. [Google Scholar]
- Makareeva, E.; Leikin, S. Chapter 7—Collagen Structure, Folding and Function. In Osteogenesis Imperfecta; Jay, R.S., Peter, H.B., Francis, H.G., Paul, D.S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 71–84. [Google Scholar]
- Guerrero, P.; Zugasti, I.; Etxabide, A.; Bao, H.N.D.; Si, T.T.; Peñalba, M.; de la Caba, K. Effect of Fructose and Ascorbic Acid on the Performance of Cross-Linked Fish Gelatin Films. Polymers 2020, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Gelatin Manufacturers of Europe. Standard Methods for the Testing of Edible Gelatine. In Gelatine Monograph, Version 17—December 2022; Gelatin Manufacturers of Europe: Brussels, Belgium, 2022; p. 45. [Google Scholar]
- Carvajal-Mena, N.; Tabilo-Munizaga, G.; Pérez-Won, M.; Lemus-Mondaca, R. Valorization of Salmon Industry by-Products: Evaluation of Salmon Skin Gelatin as a Biomaterial Suitable for 3d Food Printing. LWT 2022, 155, 112931. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kolotova, D.S.; Voron’ko, N.G.; Obluchinskaya, E.D.; Malkin, A.Y. Rheological Properties of Fish Gelatin Modified with Sodium Alginate. Polymers 2021, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- See, S.F.; Ghassem, M.; Mamot, S.; Babji, A.S. Effect of Different Pretreatments on Functional Properties of African Catfish (Clarias Gariepinus) Skin Gelatin. J. Food Sci. Technol. 2015, 52, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhou, W.; Zhang, Z.; Zhang, B. Effects of Relative Molecular Weight Distribution and Isoelectric Point on the Swelling Behavior of Gelatin Films. Front. Chem. 2022, 10, 857976. [Google Scholar] [CrossRef]
- Alfaro, A.T.; Biluca, F.C.; Marquetti, C.; Tonial, I.B.; de Souza, N.E. African Catfish (Clarias Gariepinus) Skin Gelatin: Extraction Optimization and Physical–Chemical Properties. Food Res. Int. 2014, 65, 416–422. [Google Scholar] [CrossRef]
- Enrione, J.; Char, C.; Pepczynska, M.; Padilla, C.; González-Muñoz, A.; Olguín, Y.; Quinzio, C.; Iturriaga, L.; Díaz-Calderón, P. Rheological and Structural Study of Salmon Gelatin with Controlled Molecular Weight. Polymers 2020, 12, 1587. [Google Scholar] [CrossRef]
- Casanovas, A.; Hernández, M.J.; Martí-Bonmatí, E.; Dolz, M. Cluster Classification of Dysphagia-Oriented Products Considering Flow, Thixotropy and Oscillatory Testing. Food Hydrocoll. 2011, 25, 851–859. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Xinchen, S.; Wang, H.; Zhang, L.; Sha, X.-M. Rheological and Structural Properties of Fish Scales Gelatin: Effects of Conventional and Ultrasound-Assisted Extraction. Int. J. Food Prop. 2017, 20, 1210–1220. [Google Scholar] [CrossRef]
- Zaupa, A.; Byres, N.; Zovo, C.D.; Acevedo, C.A.; Angelopoulos, I.; Terraza, C.; Nestle, N.; Abarzúa-Illanes, P.N.; Quero, F.; Díaz-Calderón, P.; et al. Cold-Adaptation of a Methacrylamide Gelatin Towards the Expansion of the Biomaterial Toolbox for Specialized Functionalities in Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.W.; Shin, M.; Lee, J.Y.; Yun, H.; Song, D.W.; Yang, Y.; Shin, B.-S.; Park, Y.H.; Lee, K.H. Fabrication of an ultrafine fish gelatin nanofibrous web from an aqueous solution by electrospinning. Int. J. Biol. Macromol. 2017, 102, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Orellana, N.; Avarias, K.; Ortiz, R.; Benavente, D.; Prieto, P. Micropatterning Technology to Design an Edible Film for In Vitro Meat Production. Food Bioprocess Technol. 2018, 11, 1267–1273. [Google Scholar] [CrossRef]
- Orellana, N.; Sánchez, E.; Benavente, D.; Prieto, P.; Enrione, J.; Acevedo, C.A. A New Edible Film to Produce In Vitro Meat. Foods 2020, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Nasri, R.; Mora, L.; Toldrá, F.; Nasri, M. Rheological and structural properties of Hemiramphus far skin gelatin: Potential use as an active fish coating agent. Food Hydrocoll. 2019, 87, 331–341. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Avena-Bustillos, R.J.; Chiou, B.; Bilbao-Sainz, C.; Bechtel, P.J.; McHugh, T.H. Ultraviolet-B Radiation Induced Cross-linking Improves Physical Properties of Cold- and Warm-Water Fish Gelatin Gels and Films. J. Food Sci. 2012, 77, E215–E223. [Google Scholar] [CrossRef]
- Eysturskarð, J.; Haug, I.J.; Elharfaoui, N.; Djabourov, M.; Draget, K.I. Structural and Mechanical Properties of Fish Gelatin as a Function of Extraction Conditions. Food Hydrocoll. 2009, 23, 1702–1711. [Google Scholar] [CrossRef]
- Martins, E.; Fernandes, R.; Alves, A.L.; Sousa, R.O.; Reis, R.L.; Silva, T.H. Skin Byproducts of Reinhardtius Hippoglossoides (Greenland Halibut) as Ecosustainable Source of Marine Collagen. Appl. Sci. 2022, 12, 11282. [Google Scholar] [CrossRef]
- ISO 6496, 1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization (ISO): Geneva, Switzerland, 1999.
- ISO 5984:2002; Animal Feeding Stuffs—Determination of Ash Content. International Organization for Standardization: Geneva, Switzerland, 2002; p. 7.
- ISO 5983-1:2005; Animal Feeding Stuffs, Determination of Nitrogen Content and Calculation of Crude Protein Content, Part 1: Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2005; p. 10.
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S. Amino Acid Determination: Methods and Techniques; M. Dekker: New York, NY, USA, 1968. [Google Scholar]
- Leach, A.A. Appendix—Notes on a Modification of the Neuman &Amp; Logan Method for the Determination of the Hydroxyproline. Biochem. J. 1960, 74, 70–71. [Google Scholar] [PubMed]
- Neuman, R.E.; Logan, M.A. The Determination of Hydroxyproline. J. Biol. Chem. 1950, 184, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- He, F. Laemmli-Sds-Page. Bio-101 2011, 1, e80. [Google Scholar] [CrossRef]




| Sample | Moisture % | Protein % | Lipid (%) | Ash (%) | Yield (%) |
|---|---|---|---|---|---|
| Skin | 86 ± 1.00 | 11.66 ± 0.65 | 0.62 ± 0.11 | 1.38 ± 0.27 | 41.4 ± 0.37 |
| Meat | 82 ± 1.4 | 7.25 ± 0.02 | 10.72 ± 0.35 | 1.01 ± 0.02 | 24 ± 0.42 |
| Frames | 86 ± 0.41 | 7.53 ± 0.32 | 3.42 ± 0.65 | 1.34 ± 0.07 | 34.6 ± 0.53 |
| Amino Acid | mg/g Freeze-Dried Skin |
|---|---|
| Alanine | 50.42 ± 10.51 |
| Glycine/arginine | 257.99 ± 52.02 |
| Aspartic acid | 44.76 ± 8.99 |
| Glutamic acid | 56.16 ± 11.44 |
| Asparagine | 0.02 ± 0.00 |
| Histidine | 4.6 ± 3.79 |
| Serin | 41.33 ± 8.97 |
| Glutamine | 0.45 ± 0.25 |
| Threonine | 24.43 ± 4.81 |
| Tyrosine | 3.6 ± 2.08 |
| α-Aminobutyric acid | 0.59 ± 0.13 |
| Methionine | 12.03 ± 2.51 |
| Valin | 15.98 ± 3.48 |
| Phenylalanin | 15.67 ± 3.52 |
| Isoleucine | 9.37 ± 1.86 |
| Leucin | 26.51 ± 5.05 |
| Lysin | 25.38 ± 5.25 |
| Hydroxyproline | 40 ± 0.09 |
| Total | 631.27 ± 119.96 |
| Amino Acids (mg/g) | G5-12-24 | G5-12-18 | G5-12-12 | G5-18-24 | G5-18-18 | G5-18-12 | G5-24-24 | G5-24-18 | G5-24-12 | G10-12-24 | G10-12-18 | G10-12-12 | G10-18-24 | G10-18-18 | G10-18-12 | G10-24-24 | G10-24-18 | G10-24-12 | G15-12-24 | G15-12-18 | G15-12-12 | G15-18-24 | G15-18-18 | G15-18-12 | G15-24-24 | G15-24-18 | G15-24-12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspartic acid | 51 ± 6 | 35 ± 9 | 49 ± 3 | 45 ± 1.5 | 44 ± 5 | 44 ± 5 | 45 ± 4 | 49 ± 3 | 44 ± 4 | 42 ± 9 | 42 ± 4 | 47 ± 10 | 44 ± 3 | 41 ± 3 | 41 ± 4 | 48 ± 6 | 44 ± 6 | 42 ± 6 | 44 ± 2 | 39 ± 8 | 53 ± 8 | 39 ± 15 | 37 ± 4 | 41 ± 4 | 44 ± 6 | 51 ± 6 | 42 ± 5 |
| Glutamic acid | 61 ± 9 | 73 ± 11.2 | 64 ± 5 | 59 ± 2 | 57 ± 7 | 58.5 ± 7 | 59 ± 6 | 63 ± 6 | 59 ± 5 | 52 ± 13 | 55 ± 4 | 61 ± 13 | 61.3 ± 4 | 54 ± 4 | 54 ± 7 | 63 ± 6 | 58 ± 8 | 55 ± 8 | 55 ± 17 | 53.6 ± 2 | 69 ± 11 | 54.54 ± 21 | 51 ± 10 | 55 ± 5 | 57 ± 8 | 67 ± 8 | 56 ± 7 |
| Histidine | 5.4 ± 1 | 2 ± 1 | 3 ± 1.4 | 7.4 ± 0.4 | 4 ± 1 | 4 ± 1 | 5 ± 3 | 3 ± 1 | 2 ± 0.9 | 1 ± 0.3 | 4 ± 0.4 | 4 ± 2 | 1.08 ± 0.05 | 4 ± 2 | 5 ± 0.1 | 3 ± 2 | 6 ± 2 | 5 ± 2 | 3 ± 2 | 4 ± 1 | 5 ± 3 | 4 ± 2 | 3 ± 1 | 5 ± 2 | 5 ± 3 | 5 ± 3 | 4 ± 2 |
| Serin | 50 ± 10 | 35.4 ± 10 | 55 ± 3 | 53 ± 2 | 50 ± 7 | 47 ± 6 | 52 ± 6 | 53 ± 5 | 49 ± 5 | 44 ± 12 | 44 ± 5 | 53 ± 12 | 44 ± 4 | 46 ± 5 | 44 ± 6 | 53 ± 6 | 51 ± 7 | 48 ± 8 | 47 ± 15 | 40 ± 10 | 65 ± 9 | 40 ± 16 | 36 ± 8 | 45 ± 6 | 50 ± 8 | 57 ± 8 | 44 ± 6 |
| Glycine + Arginine | 380 ± 41 | 230 ± 61.4 | 361 ± 23 | 323 ± 12 | 310 ± 40 | 299 ± 36 | 313 ± 34 | 355 ± 23 | 326 ± 34 | 285 ± 61 | 274 ± 27 | 346 ± 83 | 289 ± 31 | 290 ± 25 | 288 ± 33 | 351 ± 36 | 306 ± 43 | 292 ± 45 | 319 ± 103 | 254 ± 48 | 394 ± 61 | 264 ± 105 | 245 ± 26 | 291 ± 26 | 303 ± 44 | 374 ± 45 | 284 ± 41 |
| Threonine | 21 ± 3 | 20 ± 4 | 21 ± 1.5 | 25 ± 1 | 24 ± 3 | 16 ± 3 | 24.4 ± 3 | 21 ± 3 | 19 ± 2 | 21 ± 5 | 18 ± 6 | 22 ± 2 | 18 ± 6 | 22 ± 2 | 20 ± 4 | 21 ± 3 | 24 ± 3 | 23 ± 3 | 20 ± 4 | 18 ± 1 | 23 ± 3 | 20 ± 7 | 18 ± 3 | 21 ± 3 | 24 ± 4 | 22 ± 3 | 20 ± 8 |
| Tyrosine | 162 ± 16 | 103 ± 26 | 155 ± 12 | 140 ± 5 | 136 ± 15 | 136 ± 18 | 137 ± 14 | 149 ± 7 | 136 ± 11 | 125 ± 26 | 123 ± 10 | 146 ± 36 | 131 ± 9 | 128 ± 12 | 129 ± 15 | 150 ± 18 | 134 ± 16 | 127 ± 18 | 138 ± 44 | 113 ± 23 | 167 ± 28 | 116 ± 46 | 110 ± 11 | 128 ± 12 | 131 ± 19 | 158 ± 18 | 126 ± 16 |
| Alanine | 0.10 ± 0.1 | 0.54 ± 0.04 | 0.02 ± 0.002 | 1.2 ± 0.3 | 0.44 ± 0.1 | 0.51 ± 0.07 | 0.5 ± 0.08 | 0.03 ± 0.03 | 0.04 ± 0.02 | 0.01 ± 0.01 | 2 ± 0.2 | 0.03 ± 0.02 | 0.11 ± 0.03 | 0.50 ± 0.07 | 1 ± 0.08 | 0.03 ± 0.01 | 0.5 ± 0.07 | 0.20 ± 0.21 | 0.05 ± 0.01 | 0.54 ± 0.06 | 0.66 ± 0.23 | 0.98 ± 0.80 | 0.00 | 1 ± 0.05 | 1 ± 0.7 | 0.54 ± 0.08 | 1 ± 0.08 |
| Methionine | 12.5 ± 0.003 | 8.5 ± 0.05 | 12.2 ± 0.005 | 11 ± 0.1 | 12 ± 0.1 | 11 ± 0.2 | 12 ± 0.1 | 13 ± 0.01 | 11.5 ± 0.09 | 11 ± 0.03 | 9 ± 0.1 | 13 ± 0.1 | 10 ± 0.03 | 10 ± 1 | 10 ± 0.1 | 13 ± 0.8 | 11 ± 0.03 | 11 ± 0.02 | 11 ± 0.05 | 8 ± 0.05 | 14 ± 0.9 | 8 ± 0.02 | 9 ± 0.02 | 10 ± 0.1 | 11 ± 0.03 | 13 ± 0.01 | 10 ± 0.03 |
| Valin | 17 ± 2 | 12.3 ± 3 | 18 ± 1 | 153 ± 0.6 | 15 ± 0.9 | 14 ± 2 | 15 ± 0.8 | 18 ± 1 | 16 ± 1 | 16 ± 3 | 14 ± 1 | 17 ± 3.5 | 14 ± 0.4 | 13 ± 2 | 13 ± 2 | 18 ± 2 | 14 ± 0.5 | 14 ± 2 | 16 ± 3 | 12 ± 2 | 19 ± 2 | 12 ± 4 | 13 ± 1 | 13 ± 2 | 15 ± 1 | 19 ± 2 | 14 ± 2 |
| Phenylalanin | 19 ± 3 | 11 ± 4 | 17 ± 1.2 | 16.4 ± 0.5 | 15 ± 1.5 | 15 ± 2 | 16 ± 1 | 17 ± 1.5 | 15 ± 1.5 | 14 ± 4 | 16 ± 1 | 16.5 ± 4 | 14 ± 0.6 | 14 ± 2 | 15 ± 2 | 17 ± 3 | 16 ± 2 | 15 ± 2 | 15 ± 5 | 13 ± 3 | 18 ± 3 | 13 ± 5 | 12 ± 2 | 15 ± 2 | 16 ± 2 | 18 ± 2 | 15 ± 2 |
| Isoleucine | 8 ± 2 | 5.4 ± 3.5 | 8.4 ± 1.2 | 7 ± 0.6 | 7 ± 2.3 | 6 ± 1 | 7 ± 2 | 9 ± 1 | 8 ± 1.5 | 7 ± 3 | 6 ± 1 | 8 ± 4 | 6 ± 1 | 6 ± 1 | 6 ± 2 | 9 ± 1 | 6.4 ± 2 | 6 ± 2 | 7 ± 1 | 5 ± 1.6 | 9 ± 3 | 5 ± 1.4 | 5 ± 1 | 6 ± 2 | 7 ± 2 | 9 ± 2 | 6 ± 3 |
| Leucin | 22 ± 2 | 12 ± 1.4 | 20 ± 0.6 | 18 ± 0.7 | 18 ± 1 | 15 ± 1 | 19 ± 1 | 21 ± 0.6 | 19 ± 0.6 | 17 ± 2 | 16 ± 1 | 19 ± 2 | 14 ± 0.3 | 14 ± 1 | 14 ± 1 | 21 ± 1 | 19 ± 1 | 18 ± 1 | 18 ± 3 | 13 ± 1 | 22 ± 2 | 12 ± 2 | 14 ± 1 | 14 ± 0.5 | 19 ± 1 | 22 ± 1 | 15 ± 1 |
| Lysin | 29.5 ± 2.4 | 19 ± 3 | 28 ± 1.3 | 26 ± 1 | 25 ± 2.5 | 25.5 ± 2 | 25 ± 2 | 27.5 ± 1 | 25 ± 2 | 23 ± 4 | 23 ± 1.5 | 27 ± 4.5 | 24 ± 1 | 24 ± 1 | 24 ± 2 | 27 ± 2 | 24 ± 3 | 23 ± 3 | 25 ± 6 | 21 ± 3 | 30 ± 3 | 21 ± 5 | 23 ± 1 | 24 ± 1 | 25 ± 3 | 29 ± 3 | 24 ± 2 |
| Hydroxyproline | 49 ± 3 | 49 ± 5 | 53 ± 2 | 68 ± 1 | 66 ± 3.3 | 60 ± 3.4 | 65 ± 3 | 58.5 ± 2 | 50 ± 2 | 50 ± 5 | 68 ± 3 | 66 ± 6 | 68 ± 2 | 65 ± 2 | 70 ± 3 | 69 ± 3 | 69 ± 4 | 63 ± 4 | 59 ± 8 | 53 ± 5 | 63 ± 5 | 61 ± 8 | 62 ± 6 | 71 ± 2 | 66 ± 4 | 59 ± 3 | 62 ± 3 |
| Total Amino Acid | 887 ± 94 | 615 ± 155.5 | 865 ± 54.3 | 815 ± 29.5 | 783 ± 92 | 753 ± 83 | 794 ± 80 | 856 ± 54 | 777 ± 69 | 705 ± 146 | 714 ± 60 | 846 ± 179 | 738 ± 61 | 731 ± 58 | 733 ± 76 | 859 ± 84 | 782 ± 97 | 741 ± 104 | 776 ± 228 | 646 ± 119 | 946 ± 141 | 670 ± 242 | 638 ± 72 | 741 ± 67 | 773 ± 103 | 902 ± 104 | 725 ± 96 |
| Sample | Dry Matter (%) | Ash (%) |
|---|---|---|
| G10-12-18 | 89.53 ± 0.26 | 0.63 ± 0.72 |
| G10-18-12 | 91.23 ± 0.39 | 0.65 ± 0.93 |
| G10-24-24 | 89.04 ± 0.41 | 0.55 ± 0.84 |
| G10-24-18 | 90.29 ± 0.52 | 0.40 ± 0.95 |
| G15-18-12 | 89.98 ± 0.31 | 0.58 ± 0.64 |
| Gelatine Sample | Gelling Temperature (°C) | Melting Temperature (°C) | Storage Modulus (G′) (2 h, 4 °C) in Pa | Viscosity (Pa s) at 0.1 s−1 | Viscosity (Pa s) at 10 s−1 |
|---|---|---|---|---|---|
| G10-18-12 | 6.6 ± 1.2 | 14.5 ± 1.81 | 3801 ± 2.8 | 0.28 ± 0.07 | 0.17 ± 0.02 |
| G10-24-24 | 6 ± 1.61 | 14.2 ± 1.39 | 2869 ± 3.4 | 0.15 ± 0.02 | 0.11 ± 0.05 |
| G15-18-12 | 7 ± 0.95 | 15.4 ± 1.26 | 3147 ± 2.45 | 0.37 ± 0.01 | 0.24 ± 0.01 |
| Sample Code | Skin:Acid Ratio | Reaction Time (h.) | Reaction Temperature (°C) |
|---|---|---|---|
| G5-12-24 | 1:5 | 12 | 24 |
| G5-12-18 | 1:5 | 12 | 18 |
| G5-12-12 | 1:5 | 12 | 12 |
| G5-18-24 | 1:5 | 18 | 24 |
| G5-18-18 | 1:5 | 18 | 18 |
| G5-18-12 | 1:5 | 18 | 12 |
| G5-24-24 | 1:5 | 24 | 24 |
| G5-24-18 | 1:5 | 24 | 18 |
| G5-24-12 | 1:5 | 24 | 12 |
| G10-12-24 | 1:10 | 12 | 24 |
| G10-12-18 | 1:10 | 12 | 18 |
| G10-12-12 | 1:10 | 12 | 12 |
| G10-18-24 | 1:10 | 18 | 24 |
| G10-18-18 | 1:10 | 18 | 18 |
| G10-18-12 | 1:10 | 18 | 12 |
| G10-24-24 | 1:10 | 24 | 24 |
| G10-24-18 | 1:10 | 24 | 18 |
| G10-24-12 | 1:10 | 24 | 12 |
| G15-12-24 | 1:15 | 12 | 24 |
| G15-12-18 | 1:15 | 12 | 18 |
| G15-12-12 | 1:15 | 12 | 12 |
| G15-18-24 | 1:15 | 18 | 24 |
| G15-18-18 | 1:15 | 18 | 18 |
| G15-18-12 | 1:15 | 18 | 12 |
| G15-24-24 | 1:15 | 24 | 24 |
| G15-24-18 | 1:15 | 24 | 18 |
| G15-24-12 | 1:15 | 24 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasidharan, A.; Tronstad, E.R.; Rustad, T. Utilization of Lumpfish (Cyclopterus lumpus) Skin as a Source for Gelatine Extraction Using Acid Hydrolysis. Mar. Drugs 2024, 22, 169. https://doi.org/10.3390/md22040169
Sasidharan A, Tronstad ER, Rustad T. Utilization of Lumpfish (Cyclopterus lumpus) Skin as a Source for Gelatine Extraction Using Acid Hydrolysis. Marine Drugs. 2024; 22(4):169. https://doi.org/10.3390/md22040169
Chicago/Turabian StyleSasidharan, Abhilash, Elise Rabben Tronstad, and Turid Rustad. 2024. "Utilization of Lumpfish (Cyclopterus lumpus) Skin as a Source for Gelatine Extraction Using Acid Hydrolysis" Marine Drugs 22, no. 4: 169. https://doi.org/10.3390/md22040169