Hydantoanabaenopeptins from Lake Kinneret Microcystis Bloom, Isolation, and Structure Elucidation of the Possible Intermediates in the Anabaenopeptins Biosynthesis
Abstract
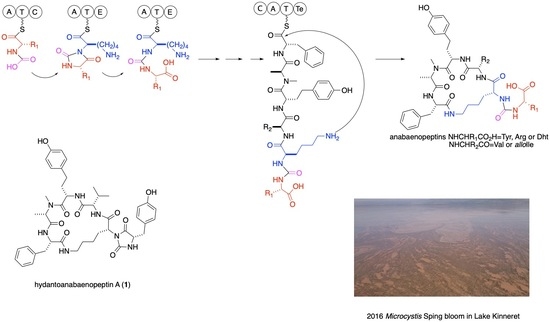
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. General Experimental Procedure
3.2. Biological Material
3.3. Isolation Procedure
3.4. Physical Data of the Compounds
3.5. Determination of the Absolute Configuration of the Amino Acids by Marfey’s Method [15]
3.6. Protease Inhibition Assays
3.6.1. Trypsin
3.6.2. Chymotrypsin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monteiro, P.R.; Amaral, S.C.D.; Siqueira, A.S.; Xavier, L.P.; Santos, A.V. Anabaenopeptins: What We Know So Far. Toxins 2021, 13, 522. [Google Scholar] [CrossRef] [PubMed]
- Zervou, S.-K.; Kaloudis, T.; Gkelis, S.; Hiskia, A.; Mazur-Marzec, H. Anabaenopeptins from Cyanobacteria in Freshwater Bodies of Greece. Toxins 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Rounge, T.B.; Rohrlack, T.; Nederbragt, A.J.; Kristensen, T.; Jakobsen, K.S. A genome-wide analysis of nonribosomal peptidesynthetase gene clusters and their peptides in a Planktothrix rubescens strain. BMC Genom. 2009, 10, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishido, T.K.; Jokela, J.; Fewer, D.P.; Wahlsten, M.; Fiore, M.F.; Sivonen, K. Simultaneous Production of Anabaenopeptins and Namalides by the Cyanobacterium Nostoc sp. CENA543. ACS Chem. Biol. 2017, 12, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Entfellner, E.; Frei, M.; Christiansen, G.; Deng, L.; Blom, J.; Kurmayer, R. Evolution of Anabaenopeptin Peptide Structural Variability in the Cyanobacterium Planktothrix. Front. Microbiol. 2017, 8, 219. [Google Scholar] [CrossRef] [Green Version]
- Lima, S.T.; Alvarenga, D.O.; Etchegaray, A.; Fewer, D.P.; Jokela, J.; Varani, A.M.; Sanz, M.; Dörr, F.A.; Pinto, E.; Sivonen, K.; et al. Genetic Organization of Anabaenopeptin and Spumigin Biosynthetic Gene Clusters in the Cyanobacterium Sphaerospermopsis torques-reginae ITEP-024. ACS Chem. Biol. 2017, 12, 769–778. [Google Scholar] [CrossRef]
- Rouhiainen, L.; Jokela, J.; Fewer, D.P.; Urmann, M.; Sivonen, K. Two Alternative Starter Modules for the Non-Ribosomal Biosynthesis of Specific Anabaenopeptin Variants in Anabaena (Cyanobacteria). Chem. Biol. 2010, 17, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, G.; Philmus, B.; Hemscheidt, T.; Kurmayer, R. Genetic Variation of Adenylation Domains of the Anabaenopeptin Synthesis Operon and Evolution of Substrate Promiscuity. J. Bacteriol. 2011, 193, 3822–3831. [Google Scholar] [CrossRef] [Green Version]
- Kaljunen, H.; Schiefelbein, S.H.H.; Stummer, D.; Kozak, S.; Meijers, R.; Christiansen, G.; Rentmeister, A. Structural Elucidation of the Bispecificity of A Domains as a Basis for Activating Non-natural Amino Acids. Angew. Chem. Int. Ed. 2015, 54, 8833–8836. [Google Scholar] [CrossRef]
- Ninio, S.; Lupu, A.; Viner-Mozzini, Y.; Zohary, T.; Sukenik, A. Multiannual variations in Microcystis bloom episodes—Temperature drives shift in species composition. Harmful Algae 2020, 92, 101710. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.N.; Alster-Gloukhovski, A.; Benyamini, Y.; Zohary, T. Lake Kinneret phytoplankton: Integrating classical and molecular taxonomy. Hydrobiologia 2016, 764, 283–302. [Google Scholar] [CrossRef]
- Elliott, T.H.; Natarajan, P.N. Infared studies of hydantoin and its derivatives. J. Pharm. Pharmacol. 1967, 19, 209–216. [Google Scholar] [CrossRef]
- Harada, K.I.; Fujii, K.; Shimada, T.; Suzuki, M.; Sano, H.; Adachi, K.; Carmichael, W.W. Two cyclic peptides, anabaenopeptins, a third group of bioactive compounds from the cyanobacterium Anabaena flos-aquae NRC 525-17. Tetrahedron Lett. 1995, 36, 1511–1514. [Google Scholar] [CrossRef]
- Shin, H.J.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Anabaenopeptins E and F, two new cyclic peptides from the cyanobacterium Oscillatoria agardhii (NIES-204). J. Nat. Prod. 1997, 60, 139–141. [Google Scholar] [CrossRef]
- Fujii, K.; Ikai, Y.; Mayumi, T.; Oka, H.; Suzuki, M.; Harada, K.-I. A Nonempirical Method Using LC/MS for Determination of the Absolute Configuration of Constituent Amino Acids in a Peptide: Elucidation of Limitations of Marfey’s Method and of Its Separation Mechanism. Anal. Chem. 1997, 69, 3346–3352. [Google Scholar] [CrossRef]
- Faris, W.M.; Safi, Z.S. Theoretical Investigation of Tautomerism Stability of Hydantoin in the Gas Phase and in Solution. Orient. J. Chem. 2014, 30, 1045–1054. [Google Scholar] [CrossRef]
- Williams, D.E.; Craig, M.; Holmes, C.F.B.; Anderson, R.J. Ferintoic Acids A and B, New Cyclic Hexpeptides from the Freshwater Cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 1996, 59, 570–575. [Google Scholar] [CrossRef]
- Zi, J.; Lantvit, D.D.; Swanson, S.M.; Orjala, J. Lyngbyaureidamides A and B, two anabaenopeptins from the cultured freshwater cyanobacterium Lyngbya sp. (SAG 36.91). Phytochemistry 2012, 74, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Reshef, V.; Carmeli, S. Schizopeptin 791, a New Anabaenopeptin-like Cyclic Peptide from the Cyanobacterium Schizothrix sp. J. Nat. Prod. 2002, 65, 1187–1189. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Harper, M.K.; Faulkner, D.J. Mozamides A and B, Cyclic Peptides from a Theonellid Sponge from Mozambique. J. Nat. Prod. 1997, 60, 779–782. [Google Scholar] [CrossRef]
- Sano, T.; Kaya, K. Oscillamide Y, A chymotrypsin Inhibitor from Toxic Oscillatoria agardhii. Tetrahedron Lett. 1995, 36, 5933–5936. [Google Scholar] [CrossRef]
- Koketsu, K.; Mitsuhashi, S.; Tabata, K. Identification of Homophenylalanine Biosynthetic Genes from the Cyanobacterium Nostoc punctiforme PCC73102 and Application to Its Microbial Production by Escherichia coli. Appl. Environ. Microbiol. 2013, 79, 2201–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreuder, H.; Liesum, A.; Lönze, P.; Stump, H.; Hoffmann, H.; Schiell, M.; Kurz, M.; Toti, L.; Bauer, A.; Kallus, C.; et al. Isolation, Co-Crystallization and Structure-Based Characterization of Anabaenopeptins as Highly Potent Inhibitors of Activated Thrombin Activatable Fibrinolysis Inhibitor (TAFIa). Sci. Rep. 2016, 6, 32958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riba, M.; Kiss-Szikszai, A.; Gonda, S.; Boros, G.; Vitál, Z.; Borsodi, A.K.; Krett, G.; Borics, G.; Ujvárosi, A.Z.; Vasas, G. Microcystis Chemotype Diversity in the Alimentary Tract of Bigheaded Carp. Toxins 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-H.; Yang, P.-C.; Lin, E.-S.; Ho, Y.-Y.; Peng, W.-F.; Lu, H.-P.; Huang, C.-C.; Huang, C.-Y. Crystal Structure of Allantoinase from Escherichia coli BL21: A Molecular Insight into a Role of the Active Site Loops in Catalysis. Molecules 2023, 28, 827. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.-H.; Kim, H.-S.; Han, K.-H.; Abendroth, J.; Niefind, K.; Schomburg, D.; Wang, J.; Kim, Y. Crystal Structure of d-Hydantoinase from Bacillus stearothermophilus: Insight into the Stereochemistry of Enantioselectivity. Biochemistry 2002, 41, 9410–9417. [Google Scholar] [CrossRef]
- Sakaue, H.; Kinouchi, T.; Fujii, N.; Takata, T. Isomeric Replacement of a Single Aspartic Acid Induces a Marked Change in Protein Function: The Example of Ribonuclease A. ACS Omega 2017, 2, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Bloudoff, K.; Schmeing, T.M. Structural and functional aspects of the nonribosomal peptide synthetase condensation domain superfamily: Discovery, dissection and diversity. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2017, 1865, 1587–1604. [Google Scholar] [CrossRef]
- Imker, H.J.; Walsh, C.T.; Wuest, W.M. SylC Catalyzes Ureido-Bond Formation During Biosynthesis of the Proteasome Inhibitor Syringolin A. J. Am. Chem. Soc. 2009, 131, 18263–18265. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.T.; O’Brien, R.V.; Khosla, C. Nonproteinogenic Amino Acid Building Blocks for Nonribosomal Peptide and Hybrid Polyketide Scaffolds. Angew. Chem. Int. Ed. 2013, 52, 7098–7124. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.; Kehr, J.-C.; Mainz, A.; Dehm, D.; Petras, D.; Süssmuth, R.D.; Dittmann, E. Biochemical Dissection of the Natural Diversification of Microcystin Provides Lessons for Synthetic Biology of NRPS. Cell Chem. Biol. 2016, 23, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Sukenik, A.; Rosin, C.; Porat, R.; Teltsch, B.; Banker, R.; Carmeli, S. Toxins from Cyanobacteria and Their Potential Impact on Water Quality of Lake Kinneret, Israel. Isr. J. Plant Sci. 1998, 46, 109–115. [Google Scholar] [CrossRef]
- Beresovsky, D.; Hadas, O.; Livne, A.; Sukenik, A.; Kaplan, A.; Carmeli, S. Toxins and Biologically Active Secondary Metabolites of Microcystis sp. isolated from Lake Kinneret. Isr. J. Chem. 2006, 46, 79–87. [Google Scholar] [CrossRef]
- Lifshitz, M.; Carmeli, S. Metabolites of a Microcystis aeruginosa bloom material from Lake Kinneret, Israel. J. Nat. Prod. 2012, 75, 209–219. [Google Scholar] [CrossRef]



| Compounds | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Position | δC | δH | δC | δH | δC | δH | δC | δH |
| Tyr/Arg-1 | 174.1, C | 174.6, C | 174.8, C | 174.1, C | ||||
| Tyr/Arg-2 | 57.0, CH | 4.34 | 55.9, CH | 4.17 | 56.0, CH | 4.14 | 57.0, CH | 4.35 |
| Tyr/Arg-2-NH | 8.55 | 8.69 | 8.74 | 8.55 | ||||
| Tyr/Arg-3a Tyr/Arg-3b | 35.5, CH2 | 2.89 2.82 | 28.8, CH2 | 1.73 1.52 | 28.9, CH2 | 1.74 1.52 | 35.5, CH2 | 2.88 2.82 |
| Tyr/Arg-4 | 124.3, C | 24.2, CH2 | 1.54 1.45 | 24.2, CH2 | 1.50 1.42 | 124.4, C | ||
| Tyr-5,5′/Arg-5 | 130.9, CH | 6.88 | 40.1, CH2 | 3.04 | 40.0, CH2 | 3.05 | 130.9, CH | 6.89 |
| Tyr-6,6′/Arg-6 -NH | 115.0, CH | 6.61 | 8.86 | 9.05 | 115.0, CH | 6.61 | ||
| Tyr/Arg-7 | 156.7, C | 156.7, C | 157.8, C | 156.6, C | ||||
| Arg-7-NH2, NH2+ | 8.43 | 8.03 | ||||||
| CO | 156.4, C | 156.9, C | 156.9, C | 156.4, C | ||||
| Lys-1 | 167.9, C | 168.2, C | 168.4, C | 168.1, C | ||||
| Lys-2 | 56.3, CH | 4.08 | 56.4, CH | 4.30 | 56.5, CH | 4.32 | 56.4, CH | 4.08 |
| Lys-3a Lys-3b | 29.3, CH2 | 2.12 0.84 | 29.6, CH2 | 2.40 1.45 | 29.6, CH2 | 2.39 1.45 | 29.3, CH2 | 2.10 0.86 |
| Lys-4a Lys-4b | 22.9, CH2 | 1.48 1.37 | 23.1, CH2 | 1.51 1.18 | 23.1, CH2 | 1.45 1.19 | 22.8, CH2 | 1.36 1.00 |
| Lys-5a Lys-5b | 28.6, CH2 | 1.37 1.30 | 28.5, CH2 | 1.50 1.40 | 28.3, CH2 | 1.51 1.46 | 28.3, CH2 | 1.39 1.34 |
| Lys-6a Lys-6b | 38.4, CH2 | 3.54 2.63 | 38.5, CH2 | 3.55 2.66 | 38.6, CH2 | 3.53 2.66 | 38.5, CH2 | 3.53 2.63 |
| Lys-6-NH | 7.41 | 7.43 | 7.46 | 7.41 | ||||
| Val/Ile-1 | 172.4, C | 172.4, C | 172.5, C | 172.5, C | ||||
| Val/Ile-2 | 57.9, CH | 3.98 | 57.9, CH | 4.09 | 56.4, CH | 4.26 | 56.4, CH | 4.15 |
| Val/Ile-2-NH | 7.76 | 7.87 | 7.89 | 7.82 | ||||
| Val/Ile-3 | 30.4, CH | 1.96 | 30.5, CH | 2.03 | 36.7, CH | 1.85 | 36.7, CH | 1.79 |
| Val/Ile-4 | 19.1, CH3 | 0.96 | 19.1, CH3 | 1.00 | 25.6, CH2 | 1.53 1.15 | 25.5, CH2 | 1.48 1.08 |
| Val/Ile-5 | 18.9, CH3 | 0.88 | 18.9, CH3 | 0.94 | 11.8, CH3 | 0.89 | 11.8, CH3 | 0.88 |
| Ile-6 | 14.8, CH3 | 0.89 | 14.8, CH3 | 0.84 | ||||
| Hty-1 | 171.2, C | 171.1, C | 171.1, C | 171.1, C | ||||
| Hty-2 | 48.9, CH | 4.74 | 48.9, CH | 4.77 | 49.0, CH | 4.77 | 49.0, CH | 4.74 |
| Hty-2-NH | 8.97 | 8.98 | 8.98 | 8.92 | ||||
| Hty-3a Hty-3b | 33.5, CH2 | 1.85 1.73 | 33.4, CH2 | 1.85 1.74 | 33.4, CH2 | 1.85 1.74 | 33.4, CH2 | 1.85 1.72 |
| Hty-4a Hty-4b | 30.7, CH2 | 2.60 2.40 | 30.7, CH2 | 2.61 2.42 | 30.8, CH2 | 2.62 2.42 | 30.7, CH2 | 2.59 2.40 |
| Hty-5 | 131.0, C | 131.1, C | 131.0, C | 131.1, C | ||||
| Hty-6,6′ | 129.2, CH | 6.98 | 129.2, CH | 6.98 | 129.1, CH | 6.98 | 129.2, CH | 6.97 |
| Hty-7,7′ | 115.3, CH | 6.66 | 115.3, CH | 6.66 | 115.4, CH | 6.66 | 115.3, CH | 6.65 |
| Hty-8 | 155.9, C | 155.8, C | 155.9, C | 155.8, C | ||||
| NMe-Ala-1 | 170.0, C | 170.0, C | 170.0, C | 170.0, C | ||||
| NMe-Ala-2 | 54.5, CH | 4.85 | 54.6, CH | 4.87 | 54.6, CH | 4.88 | 54.5, CH | 4.85 |
| NMe-Ala-3 | 14.1, CH3 | 1.05 | 14.1, CH3 | 1.07 | 14.1, CH3 | 1.07 | 14.1, CH3 | 1.06 |
| NMe-Ala-Me | 27.2, CH3 | 1.78 | 27.3, CH3 | 1.79 | 27.3, CH3 | 1.81 | 27.3, CH3 | 1.79 |
| Phe-1 | 170.9, C | 171.2, C | 171.3, C | 171.2, C | ||||
| Phe-2 | 55.1, CH | 4.34 | 55.1, CH | 4.36 | 55.2, CH | 4.35 | 55.2, CH | 4.35 |
| Phe-2-NH | 8.69 | 8.70 | 8.65 | 8.65 | ||||
| Phe-3a Phe-3b | 37.7, CH2 | 3.23 2.81 | 37.7, CH2 | 3.23 2.83 | 37.7, CH2 | 3.23 2.84 | 37.7, CH2 | 3.24 2.82 |
| Phe-4 | 138.5, C | 138.4, C | 138.5, C | 138.5, C | ||||
| Phe-5,5′ | 129.1, CH | 7.06 | 129.0, CH | 7.07 | 129.1, CH | 7.07 | 129.1, CH | 7.07 |
| Phe-6,6′ | 128.5, CH | 7.19 | 128.5, CH | 7.19 | 128.4, CH | 7.19 | 128.4, CH | 7.19 |
| Phe-7 | 126.3, CH | 7.14 | 126.3, CH | 7.14 | 126.3, CH | 7.14 | 126.3, CH | 7.16 |
| Compounds | 5 | 6 | ||
|---|---|---|---|---|
| Position | δC | δH | δC | δH |
| Dht-1 | 174.6, C | 174.0, C | ||
| Dht-2 | 53.3, CH | 3.91, m | 56.2, CH | 4.15, m |
| Dht-2-NH | 6.27, brd | 8.61, brs | ||
| Dht-3a Dht-3b | 32.3, CH2 | 1.61, m 1.50, m | 30.8, CH2 | 1.66, m 1.50, m |
| Dht-4a Dht-4b | 27.7, CH2 | 1.48, m 1.45, m | 28.9, CH2 | 1.48, m 1.45, m |
| Dht-5 | 34.4, CH2 | 2.40, m | 34.0, CH2 | 2.42, m |
| Dht-6 | 132.4, C | 131.6, C | ||
| Dht-7,7′ | 129.2, CH | 6.92, d | 129.3, CH | 6.92, d |
| Dht-8,8′ | 115.2, CH | 6.63, d | 115.2, CH | 6.65, d |
| Dht-9 | 155.4, C | 155.9, C | ||
| CO | 157.4, C | 156.9, C | ||
| Lys-1 | 172.7, C | 168.4, C | ||
| Lys-2 | 55.0, CH | 3.91, ddd | 56.5, CH | 4.31, m |
| Lys-2-NH | 6.48, brd | - | ||
| Lys-3 | 32.1, CH2 | 1.60, m | 29.6, CH2, | 2.39, m 1.44, m |
| Lys-4a Lys-4b | 20.6, CH3 | 1.28, m 1.16, m | 23.1, CH2 | 1.45, m 1.17, m |
| Lys-5 | 28.3, CH2 | 1.41, m | 28.6, CH2 | 1.48, m 1.38, m |
| Lys-6a Lys-6b | 38.5, CH2 | 3.56, m 2.78, m | 38.5, CH2 | 3.53, m 2.65, m |
| Lys-6-NH | 7.14, m | 7.43, t | ||
| Val-1 | 172.9, C | 172.4, C | ||
| Val-2 | 58.4, CH | 3.88, dd | 57.9, CH | 4.08, t |
| Val-2-NH | 7.06, m | 7.88, d | ||
| Val-3 | 30.2, CH2 | 1.95, dqq | 30.5, CH | 2.01, m |
| Val-4 | 19.6, CH3 | 0.92, d | 19.2, CH3 | 0.99, d |
| Val-5 | 19.1, CH3 | 1.02, d | 18.8, CH3 | 0.92, d |
| Hty-1 | 171.1, C | 171.2, C | ||
| Hty-2 | 48.9, CH | 4.71, dt | 49.0, CH | 4.77, m |
| Hty-2-NH | 8.92, d | 8.96, brs | ||
| Hty-3a Hty-3b | 33.5, CH2 | 1.85, m 1.70, m | 33.4, CH2 | 1.85, m 1.72, m |
| Hty-4a Hty-4b | 30.7, CH2 | 2.62, m 2.41, m | 30.7, CH2 | 2.60, m 2.40, m |
| Hty-5 | 131.2, C | 131.0, C | ||
| Hty-6,6′ | 129.3, CH | 7.00, d | 129.2, CH | 6.98, d |
| Hty-7,7′ | 115.4, CH | 6.65, d | 115.4, CH | 6.66, d |
| Hty-8 | 155.8, C | 155.7, C | ||
| NMe-Ala-1 | 170.1, C | 170.0, C | ||
| NMe-Ala-2 | 54.5, CH | 4.77, q | 54.6, CH | 4.87, q |
| NMe-Ala-3 | 14.1, CH3 | 1.05, d | 14.1, CH3 | 1.06, d |
| NMe-Ala-Me | 27.3, CH3 | 1.76, s | 27.3, CH3 | 1.79, s |
| Phe-1 | 171.2, C | 171.1, C | ||
| Phe-2 | 55.2, CH | 4.36, ddd | 55.2, CH | 4.35, ddd |
| Phe-2-NH | 8.68, d | 8.69, d | ||
| Phe-3a Phe-3b | 37.7, CH2 | 3.30, m 2.77, dd | 37.7, CH2 | 3.23, m 2.81, dd |
| Phe-4 | 138.5, C | 138.4, C | ||
| Phe-5,5′ | 129.1, CH | 7.05, d | 129.1, CH | 7.07, d |
| Phe-6,6′ | 128.5, CH | 7.18, dd | 128.5, CH | 7.19, t |
| Phe-7 | 126.3, CH | 7.13, t | 126.3, CH | 7.14, t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weisthal Algor, S.; Sukenik, A.; Carmeli, S. Hydantoanabaenopeptins from Lake Kinneret Microcystis Bloom, Isolation, and Structure Elucidation of the Possible Intermediates in the Anabaenopeptins Biosynthesis. Mar. Drugs 2023, 21, 401. https://doi.org/10.3390/md21070401
Weisthal Algor S, Sukenik A, Carmeli S. Hydantoanabaenopeptins from Lake Kinneret Microcystis Bloom, Isolation, and Structure Elucidation of the Possible Intermediates in the Anabaenopeptins Biosynthesis. Marine Drugs. 2023; 21(7):401. https://doi.org/10.3390/md21070401
Chicago/Turabian StyleWeisthal Algor, Shira, Assaf Sukenik, and Shmuel Carmeli. 2023. "Hydantoanabaenopeptins from Lake Kinneret Microcystis Bloom, Isolation, and Structure Elucidation of the Possible Intermediates in the Anabaenopeptins Biosynthesis" Marine Drugs 21, no. 7: 401. https://doi.org/10.3390/md21070401