Fibrin and Marine-Derived Agaroses for the Generation of Human Bioartificial Tissues: An Ex Vivo and In Vivo Study
Abstract
:1. Introduction
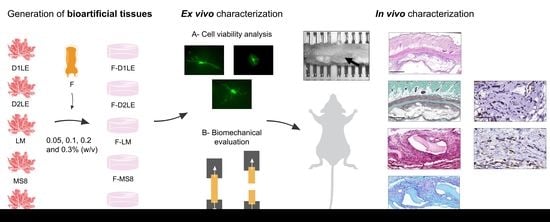
2. Results
2.1. Cell Viability of Human Fibroblasts Immersed within Fibrin-Agarose Biomaterials
2.2. Biomechanical Properties of Fibrin-Agarose Biomaterials
2.3. Systemic Effects of Each Type of Biomaterial Grafted In Vivo in Laboratory Rats
2.4. In Situ Morphological Analysis of Biomaterials Grafted In Vivo in Laboratory Rats
2.5. Histochemical and Immunohistochemical Analysis of Biomaterials Grafted In Vivo in Laboratory Rats
3. Discussion
3.1. All Combinations of Fibrin and Marine-Derived Agaroses Hydrogels Are Highly Biocompatible Ex Vivo
3.2. The Agarose Type and Concentration Influence the Stiffness and Elasticity of the Resulting Hydrogels
3.3. All Types of FA Biomaterials Were Safe When Implanted In Vivo and Showed Different Biointegration Rates at the Implant Site
4. Materials and Methods
4.1. Cell Cultures
4.2. Generation of Bioartificial Tissues Using Fibrin and Fibrin-Agarose Biomaterials
4.3. Cell Viability Analysis
4.4. Biomechanical Evaluation
4.5. In Vivo Analysis
4.6. Histology, Histochemistry and Immunohistochemistry
4.7. Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Shen, X.; Ma, R.; Hou, Y.; Qian, Y.; Fan, C. Biological and Biocompatible Characteristics of Fullerenols Nanomaterials for Tissue Engineering. Histol. Histopathol. 2021, 36, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Yoo, J.J.; Atala, A. Solid Organ Bioprinting: Strategies to Achieve Organ Function. Chem. Rev. 2020, 120, 11093–11127. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Campbell Ritchie, A.; Everitt, N.M. Recombinant Human Collagen/Chitosan-Based Soft Hydrogels as Biomaterials for Soft Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111846. [Google Scholar] [CrossRef] [PubMed]
- Brassolatti, P.; Bossini, P.S.; de Andrade, A.L.M.; Luna, G.L.F.; da Silva, J.V.; Almeida-Lopes, L.; Napolitano, M.A.; da Avó, L.R.S.; de Leal, Â.M.O.; de Anibal, F.F. Comparison of Two Different Biomaterials in the Bone Regeneration (15, 30 and 60 Days) of Critical Defects in Rats. Acta Cir. Bras. 2021, 36, e360605. [Google Scholar] [CrossRef] [PubMed]
- Johari, N.; Khodaei, A.; Samadikuchaksaraei, A.; Reis, R.L.; Kundu, S.C.; Moroni, L. Ancient Fibrous Biomaterials from Silkworm Protein Fibroin and Spider Silk Blends: Biomechanical Patterns. Acta Biomater. 2022, 153, 38–67. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for Tissue Engineering and 3D Cell Culture. Methods Mol. Biol. 2011, 695, 17–39. [Google Scholar] [CrossRef]
- Mokhtari-Jafari, F.; Amoabediny, G.; Dehghan, M.M. Role of Biomechanics in Vascularization of Tissue-Engineered Bones. J. Biomech. 2020, 110, 109920. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Paggi, U.; Zhang, X.-Y.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; et al. Additively Manufactured Functionally Graded Biodegradable Porous Iron. Acta Biomater. 2019, 96, 646–661. [Google Scholar] [CrossRef]
- Kim, W.; Ferguson, V.L.; Borden, M.; Neu, C.P. Application of Elastography for the Noninvasive Assessment of Biomechanics in Engineered Biomaterials and Tissues. Ann. Biomed. Eng. 2016, 44, 705–724. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Woo, K.M. Fibrin-Based Biomaterial Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1064, 253–261. [Google Scholar] [CrossRef]
- Mosesson, M.W. Fibrinogen and Fibrin Structure and Functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Murillo, J.A.; Simental-Mendía, M.A.; Moncada-Saucedo, N.K.; Delgado-Gonzalez, P.; Islas, J.F.; Roacho-Pérez, J.A.; Garza-Treviño, E.N. Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair. Int. J. Mol. Sci. 2022, 23, 9879. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; O’Brien, T.; Pandit, A. Fibrin as a Delivery System for Therapeutic Drugs and Biomolecules. Tissue Eng. Part B Rev. 2009, 15, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, M.C.; Felice, F.; Balbarini, A.; Di Stefano, R. Fibrin as a Scaffold for Cardiac Tissue Engineering. Biotechnol. Appl. Biochem. 2011, 58, 301–310. [Google Scholar] [CrossRef]
- Cassaro, C.V.; Justulin, L.A.; de Lima, P.R.; de Golim, M.A.; Biscola, N.P.; de Castro, M.V.; de Oliveira, A.L.R.; Doiche, D.P.; Pereira, E.J.; Ferreira, R.S.; et al. Fibrin Biopolymer as Scaffold Candidate to Treat Bone Defects in Rats. J. Venom Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190027. [Google Scholar] [CrossRef] [Green Version]
- Barbon, S.; Stocco, E.; Macchi, V.; Contran, M.; Grandi, F.; Borean, A.; Parnigotto, P.P.; Porzionato, A.; De Caro, R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1701. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Lyu, C.; Teng, L.; Xie, X.; Sun, J.; Zou, D.; Lu, J. An Injectable Fibrin Scaffold Rich in Growth Factors for Skin Repair. Biomed. Res. Int. 2021, 2021, 8094932. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A Review of Fibrin and Fibrin Composites for Bone Tissue Engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Ma, X.; Hu, H. Marine Polysaccharides as a Versatile Biomass for the Construction of Nano Drug Delivery Systems. Mar. Drugs 2021, 19, 345. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Irastorza-Lorenzo, A.; Sánchez-Porras, D.; Ortiz-Arrabal, O.; de Frutos, M.J.; Esteban, E.; Fernández, J.; Janer, A.; Campos, A.; Campos, F.; Alaminos, M. Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 1923. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Sivashankari, P.R.; Prabaharan, M. Three-Dimensional Porous Scaffolds Based on Agarose/Chitosan/Graphene Oxide Composite for Tissue Engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Moldovan, L.; Constantin, D.; Stanciuc, A.M.; Sarbu Boeti, P.M.; Efrimescu, I.C. Collagen-Based Scaffolds for Skin Tissue Engineering. J. Med. Life 2011, 4, 172–177. [Google Scholar]
- Alaminos, M.; Del Carmen Sánchez-Quevedo, M.; Muñoz-Avila, J.I.; Serrano, D.; Medialdea, S.; Carreras, I.; Campos, A. Construction of a Complete Rabbit Cornea Substitute Using a Fibrin-Agarose Scaffold. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3311–3317. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Elices, C.; Chato-Astrain, J.; Oyonarte, S.; Bermejo-Casares, F.; España-López, A.; Fernández-Valadés, R.; Sánchez-Quevedo, M.D.C.; Alaminos, M.; Martín-Piedra, M.A.; Garzón, I. Generation of a Novel Model of Bioengineered Human Oral Mucosa with Increased Vascularization Potential. J. Periodontal Res. 2021, 56, 1116–1131. [Google Scholar] [CrossRef]
- Carriel, V.; Garzón, I.; Jiménez, J.-M.; Oliveira, A.-C.-X.; Arias-Santiago, S.; Campos, A.; Sánchez-Quevedo, M.-C.; Alaminos, M. Epithelial and Stromal Developmental Patterns in a Novel Substitute of the Human Skin Generated with Fibrin-Agarose Biomaterials. Cells Tissues Organs 2012, 196, 1–12. [Google Scholar] [CrossRef]
- Carriel, V.; Scionti, G.; Campos, F.; Roda, O.; Castro, B.; Cornelissen, M.; Garzón, I.; Alaminos, M. In Vitro Characterization of a Nanostructured Fibrin Agarose Bio-Artificial Nerve Substitute. J. Tissue Eng. Regen. Med. 2017, 11, 1412–1426. [Google Scholar] [CrossRef] [Green Version]
- González-Quevedo, D.; Sánchez-Porras, D.; García-García, Ó.-D.; Chato-Astrain, J.; Díaz-Ramos, M.; Campos, A.; Carriel, V.; Campos, F. Nanostructured Fibrin-Based Hydrogel Membranes for Use as an Augmentation Strategy in Achilles Tendon Surgical Repair in Rats. Eur. Cell Mater. 2022, 43, 162–178. [Google Scholar] [CrossRef]
- Carriel, V.; Vizcaíno-López, G.; Chato-Astrain, J.; Durand-Herrera, D.; Alaminos, M.; Campos, A.; Sánchez-Montesinos, I.; Campos, F. Scleral Surgical Repair through the Use of Nanostructured Fibrin/Agarose-Based Films in Rabbits. Exp. Eye Res. 2019, 186, 107717. [Google Scholar] [CrossRef]
- Ionescu, A.-M.; Alaminos, M.; de la Cruz Cardona, J.; de Dios García-López Durán, J.; González-Andrades, M.; Ghinea, R.; Campos, A.; Hita, E.; del Mar Pérez, M. Investigating a Novel Nanostructured Fibrin-Agarose Biomaterial for Human Cornea Tissue Engineering: Rheological Properties. J. Mech. Behav. Biomed. Mater. 2011, 4, 1963–1973. [Google Scholar] [CrossRef]
- Scionti, G.; Moral, M.; Toledano, M.; Osorio, R.; Durán, J.D.G.; Alaminos, M.; Campos, A.; López-López, M.T. Effect of the Hydration on the Biomechanical Properties in a Fibrin-Agarose Tissue-like Model. J. Biomed. Mater. Res. A 2014, 102, 2573–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, F.; Bonhome-Espinosa, A.B.; Chato-Astrain, J.; Sánchez-Porras, D.; García-García, Ó.D.; Carmona, R.; López-López, M.T.; Alaminos, M.; Carriel, V.; Rodriguez, I.A. Evaluation of Fibrin-Agarose Tissue-Like Hydrogels Biocompatibility for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- González-Andrades, M.; Mata, R.; González-Gallardo, M.D.C.; Medialdea, S.; Arias-Santiago, S.; Martínez-Atienza, J.; Ruiz-García, A.; Pérez-Fajardo, L.; Lizana-Moreno, A.; Garzón, I.; et al. A Study Protocol for a Multicentre Randomised Clinical Trial Evaluating the Safety and Feasibility of a Bioengineered Human Allogeneic Nanostructured Anterior Cornea in Patients with Advanced Corneal Trophic Ulcers Refractory to Conventional Treatment. BMJ Open 2017, 7, e016487. [Google Scholar] [CrossRef]
- Egea-Guerrero, J.J.; Carmona, G.; Correa, E.; Mata, R.; Arias-Santiago, S.; Alaminos, M.; Gacto, P.; Cuende, N. Transplant of Tissue-Engineered Artificial Autologous Human Skin in Andalusia: An Example of Coordination and Institutional Collaboration. Transplant. Proc. 2019, 51, 3047–3050. [Google Scholar] [CrossRef] [PubMed]
- González-Quevedo, D.; Díaz-Ramos, M.; Chato-Astrain, J.; Sánchez-Porras, D.; Tamimi, I.; Campos, A.; Campos, F.; Carriel, V. Improving the Regenerative Microenvironment during Tendon Healing by Using Nanostructured Fibrin/Agarose-Based Hydrogels in a Rat Achilles Tendon Injury Model. Bone Jt. J. 2020, 102-B, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Quan, L.; Ao, Q. Characteristics of Marine Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2022, 20, 372. [Google Scholar] [CrossRef] [PubMed]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New Insight into Agarose Gel Mechanical Properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Zenhausern, F. The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. [Google Scholar] [CrossRef] [PubMed]
- Cioroianu, A.R.; Storm, C. Normal Stresses in Elastic Networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2013, 88, 052601. [Google Scholar] [CrossRef] [Green Version]
- Maher, M.; Glattauer, V.; Onofrillo, C.; Duchi, S.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Suitability of Marine- and Porcine-Derived Collagen Type I Hydrogels for Bioprinting and Tissue Engineering Scaffolds. Mar. Drugs 2022, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Rico-Sánchez, L.; Garzón, I.; González-Andrades, M.; Ruíz-García, A.; Punzano, M.; Lizana-Moreno, A.; Muñoz-Ávila, J.I.; Sánchez-Quevedo, M.D.C.; Martínez-Atienza, J.; Lopez-Navas, L.; et al. Successful Development and Clinical Translation of a Novel Anterior Lamellar Artificial Cornea. J. Tissue Eng. Regen. Med. 2019, 13, 2142–2154. [Google Scholar] [CrossRef] [Green Version]
- Benayahu, D.; Pomeraniec, L.; Shemesh, S.; Heller, S.; Rosenthal, Y.; Rath-Wolfson, L.; Benayahu, Y. Biocompatibility of a Marine Collagen-Based Scaffold In Vitro and In Vivo. Mar. Drugs 2020, 18, 420. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- Corradetti, B.; Taraballi, F.; Corbo, C.; Cabrera, F.; Pandolfi, L.; Minardi, S.; Wang, X.; Van Eps, J.; Bauza, G.; Weiner, B.; et al. Immune Tuning Scaffold for the Local Induction of a Pro-Regenerative Environment. Sci. Rep. 2017, 7, 17030. [Google Scholar] [CrossRef] [Green Version]
- Klopfleisch, R. Macrophage Reaction against Biomaterials in the Mouse Model—Phenotypes, Functions and Markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef]
- Isali, I.; McClellan, P.; Wong, T.R.; Cingireddi, S.; Jain, M.; Anderson, J.M.; Hijaz, A.; Akkus, O. In Vivo Delivery of M0, M1, and M2 Macrophage Subtypes via Genipin-Cross-Linked Collagen Biotextile. Tissue Eng. Part A 2022, 28, 672–684. [Google Scholar] [CrossRef]
- Welc, S.S.; Wehling-Henricks, M.; Antoun, J.; Ha, T.T.; Tous, I.; Tidball, J.G. Differential Effects of Myeloid Cell PPARδ and IL10 in Regulating Macrophage Recruitment, Phenotype and Regeneration Following Acute Muscle Injury. J. Immunol. 2020, 205, 1664–1677. [Google Scholar] [CrossRef]
- Warren, G.L.; Hulderman, T.; Mishra, D.; Gao, X.; Millecchia, L.; O’Farrell, L.; Kuziel, W.A.; Simeonova, P.P. Chemokine Receptor CCR2 Involvement in Skeletal Muscle Regeneration. FASEB J. 2005, 19, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Battiston, K.G.; Labow, R.S.; Simmons, C.A.; Santerre, J.P. Generating Favorable Growth Factor and Protease Release Profiles to Enable Extracellular Matrix Accumulation within an in Vitro Tissue Engineering Environment. Acta Biomater. 2017, 54, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Chato-Astrain, J.; Chato-Astrain, I.; Sánchez-Porras, D.; García-García, Ó.-D.; Bermejo-Casares, F.; Vairo, C.; Villar-Vidal, M.; Gainza, G.; Villullas, S.; Oruezabal, R.-I.; et al. Generation of a Novel Human Dermal Substitute Functionalized with Antibiotic-Loaded Nanostructured Lipid Carriers (NLCs) with Antimicrobial Properties for Tissue Engineering. J. Nanobiotechnol. 2020, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Rodríguez, C.-A.; Garzón, I.; Garrido-Gómez, J.; Oliveira, A.-C.-X.; Martín-Piedra, M.-Á.; Scionti, G.; Carriel, V.; Hernández-Cortés, P.; Campos, A.; Alaminos, M. Identification of Histological Patterns in Clinically Affected and Unaffected Palm Regions in Dupuytren’s Disease. PLoS ONE 2014, 9, e112457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.C.; Garzón, I.; Ionescu, A.M.; Carriel, V.; de la Cardona, J.C.; González-Andrades, M.; Pérez, M.d.M.; Alaminos, M.; Campos, A. Evaluation of Small Intestine Grafts Decellularization Methods for Corneal Tissue Engineering. PLoS ONE 2013, 8, e66538. [Google Scholar] [CrossRef] [Green Version]
- Carriel, V.; Garzón, I.; Campos, A.; Cornelissen, M.; Alaminos, M. Differential Expression of GAP-43 and Neurofilament during Peripheral Nerve Regeneration through Bio-Artificial Conduits. J. Tissue Eng. Regen. Med. 2017, 11, 553–563. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Arrabal, O.; Irastorza-Lorenzo, A.; Campos, F.; Martín-Piedra, M.Á.; Carriel, V.; Garzón, I.; Ávila-Fernández, P.; de Frutos, M.J.; Esteban, E.; Fernández, J.; et al. Fibrin and Marine-Derived Agaroses for the Generation of Human Bioartificial Tissues: An Ex Vivo and In Vivo Study. Mar. Drugs 2023, 21, 187. https://doi.org/10.3390/md21030187
Ortiz-Arrabal O, Irastorza-Lorenzo A, Campos F, Martín-Piedra MÁ, Carriel V, Garzón I, Ávila-Fernández P, de Frutos MJ, Esteban E, Fernández J, et al. Fibrin and Marine-Derived Agaroses for the Generation of Human Bioartificial Tissues: An Ex Vivo and In Vivo Study. Marine Drugs. 2023; 21(3):187. https://doi.org/10.3390/md21030187
Chicago/Turabian StyleOrtiz-Arrabal, Olimpia, Ainhoa Irastorza-Lorenzo, Fernando Campos, Miguel Ángel Martín-Piedra, Víctor Carriel, Ingrid Garzón, Paula Ávila-Fernández, María José de Frutos, Emilio Esteban, Javier Fernández, and et al. 2023. "Fibrin and Marine-Derived Agaroses for the Generation of Human Bioartificial Tissues: An Ex Vivo and In Vivo Study" Marine Drugs 21, no. 3: 187. https://doi.org/10.3390/md21030187