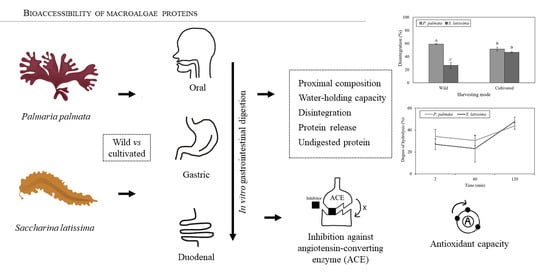
In Vitro Bioaccessibility of Proteins and Bioactive Compounds of Wild and Cultivated Seaweeds from the Gulf of Saint Lawrence
Abstract
:1. Introduction
2. Results
2.1. Water Holding Capacity of Seaweeds
2.2. In Vitro Digestion Model
2.3. Undigested Nutrients
2.4. Bioactivities
3. Discussion
3.1. Sample Characterization
3.2. In Vitro Digestion Model
3.3. Undigested Nutrients
3.4. Bioactivity
4. Materials and Methods
4.1. Chemicals
4.2. Sample Characterization
4.3. In Vitro Digestion Model
(Protein content in seaweed before digestion (mg))) × 100,
4.4. Undigested Nutrients
dry pellets)/(Initial weight of dry seaweeds before digestion)) × 100
4.5. Bioactivities
4.5.1. ACE Inhibitory Activity
4.5.2. Antioxidant Capacity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The Protein Content of Seaweeds: A Universal Nitrogen-to-Protein Conversion Factor of Five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed proteins. In Proteins in Food Processing, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 245–262. ISBN 9780081007297. [Google Scholar]
- Pereira, L. A Review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 15–47. ISBN 978−1614708780. [Google Scholar]
- Fleurence, J.L. Seaweed Proteins: Biochemical, Nutritional Aspects and Potential Uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I.; Hernández-Ledesma, B. Antihypertensive Peptides from Food Proteins: A Review. Food Funct. 2012, 3, 350–361. [Google Scholar] [CrossRef]
- Kandale, A.; Meena, A.K.; Panda, P. Medicinal Plant Used in Indian System of Medicine View Project Treatment of Heavy Metal Contaminated Waste Water Using Different Techniques View Project. J. Pharm. Res. 2011, 4, 219–221. [Google Scholar]
- Fujiwara-Arasaki, T.; Mino, N.; Kuroda, M. The protein value in human nutrition of edible marine algae in Japan. In Eleventh International Seaweed Symposium; Bird, C.J., Ragan, M.A., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1984; Volume 22, pp. 513–516. [Google Scholar]
- Juul, L.; Stødkilde, L.; Ingerslev, A.K.; Bruhn, A.; Jensen, S.K.; Dalsgaard, T.K. Digestibility of Seaweed Protein from Ulva Sp. and Saccharina Latissima in Rats. Algal Res. 2022, 63, 102644. [Google Scholar] [CrossRef]
- Mišurcová, L. Seaweed digestibility and methods used for digestibility determination. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 285–301. ISBN 9780470979181. [Google Scholar]
- Mišurcová, L.; Kráčmar, S.; Klejdus, B.; Vacek, J. Nitrogen Content, Dietary Fiber, and Digestibility in Algal Food Products. Czech J. Food Sci. 2010, 28, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Demarco, M.; Oliveira de Moraes, J.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, Bioaccessibility and Bioactivity of Compounds from Algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Intawongse, M.; Kongchouy, N.; Dean, J.R. Bioaccessibility of Heavy Metals in the Seaweed Caulerpa Racemosa Var. Corynephora: Human Health Risk from Consumption. Instrum. Sci. Technol. 2018, 46, 628–644. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, R.D.; Hortas, V.R.; Bermejo Barrera, P. In vivo and in vitro studies of seaweed compounds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 348–355. ISBN 9780470979181. [Google Scholar]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In Vitro Human Digestion Models for Food Applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Goñi, I.; Gudiel-Urbano, M.; Saura-Calixto, F. In Vitro Determination of Digestible and Unavailable Protein in Edible Seaweeds. J. Sci. Food Agric. 2002, 82, 1850–1854. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional Value of Proteins from Edible Seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef] [PubMed]
- De Bhowmick, G.; Hayes, M. In Vitro Protein Digestibility of Selected Seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef]
- Yabuta, Y.; Fujimura, H.; Shil Kwak, C.; Enomoto, T.; Watanabe, F. Antioxidant Activity of the Phycoerythrobilin Compound Formed from a Dried Korean Purple Laver (Porphyra Sp.) during in Vitro Digestion. Food Sci. Technol. Res. 2010, 16, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Fleurence, J.; Chenard, E.; Luçon, M. Determination of the Nutritional Value of Proteins Obtained from Ulva Armoricana. J. Appl. Phycol. 1999, 11, 231–239. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.K. Nutritional Evaluation of Some Subtropical Red and Green Seaweeds Part I—Proximate Composition, Amino Acid Profiles and Some Physico-Chemical Properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional Quality of Some Wild and Cultivated Seaweeds: Nutrient Composition, Total Phenolic Content and in Vitro Digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Machů, L.; Mišurcová, L.; Samek, D.; Hrabě, J.; Fišera, M. In Vitro Digestibility of Different Commercial Edible Algae Products. J. Aquat. Food Prod. Technol. 2014, 23, 423–435. [Google Scholar] [CrossRef]
- Rozan, P.; Lamghari, R.; Linder, M.; Villaume, C.; Fanni, J.; Parmentier, M.; Méjean, L. In Vivo and in Vitro Digestibility of Soybean, Lupine, and Rapeseed Meal Proteins after Various Technological Processes. J. Agric. Food Chem. 1997, 45, 1762–1769. [Google Scholar] [CrossRef]
- Afonso, C.; Matos, J.; Guarda, I.; Gomes-Bispo, A.; Gomes, R.; Cardoso, C.; Gueifão, S.; Delgado, I.; Coelho, I.; Castanheira, I.; et al. Bioactive and Nutritional Potential of Alaria Esculenta and Saccharina Latissima. J. Appl. Phycol. 2021, 33, 501–513. [Google Scholar] [CrossRef]
- Beaulieu, L.; Sirois, M.; Tamigneaux, É. Evaluation of the in Vitro Biological Activity of Protein Hydrolysates of the Edible Red Alga, Palmaria palmata (Dulse) Harvested from the Gaspe Coast and Cultivated in Tanks. J. Appl. Phycol. 2016, 28, 3101–3115. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino Acids, Fatty Acids, and Dietary Fibre in Edible Seaweed Products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Marinho, G.S.; Holdt, S.L.; Angelidaki, I. Seasonal Variations in the Amino Acid Profile and Protein Nutritional Value of Saccharina Latissima Cultivated in a Commercial IMTA System. J. Appl. Phycol. 2015, 27, 1991–2000. [Google Scholar] [CrossRef]
- De la Moneda, A.; Carro, M.D.; Weisbjerg, M.R.; Roleda, M.Y.; Lind, V.; Novoa-Garrido, M.; Molina-Alcaide, E. Variability and Potential of Seaweeds as Ingredients of Ruminant Diets: An in Vitro Study. Animals 2019, 9, 851. [Google Scholar] [CrossRef] [Green Version]
- McCauley, J.I.; Winberg, P.C.; Meyer, B.J.; Skropeta, D. Effects of Nutrients and Processing on the Nutritionally Important Metabolites of Ulva Sp. (Chlorophyta). Algal Res. 2018, 35, 586–594. [Google Scholar] [CrossRef]
- Vasconcelos, M.M.M.; Marson, G.V.; Turgeon, S.L.; Tamigneaux, E.; Beaulieu, L. Environmental Conditions Influence on the Physicochemical Properties of Wild and Cultivated Palmaria palmata in the Canadian Atlantic Shore. J. Appl. Phycol. 2022, 34, 2565–2578. [Google Scholar] [CrossRef]
- Olischläger, M.; Iñiguez, C.; Gordillo, F.J.L.; Wiencke, C. Biochemical Composition of Temperate and Arctic Populations of Saccharina Latissima after Exposure to Increased PCO2 and Temperature Reveals Ecotypic Variation. Planta 2014, 240, 1213–1224. [Google Scholar] [CrossRef] [Green Version]
- Lafeuille, B.; Francezon, N.; Goulet, C.; Perreault, V.; Turgeon, S.L.; Beaulieu, L. Impact of Temperature and Cooking Time on the Physicochemical Properties and Sensory Potential of Seaweed Water Extracts of Palmaria palmata and Saccharina Longicruris. J. Appl. Phycol. 2022, 34, 1731–1747. [Google Scholar] [CrossRef]
- Rioux, L.E.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A Traditional Ingredients for New Gastronomic Sensation. Food Hydrocoll. 2017, 68, 255–265. [Google Scholar] [CrossRef]
- Bondu, S.; Bonnet, C.; Gaubert, J.; Deslandes, É.; Turgeon, S.L.; Beaulieu, L. Bioassay-Guided Fractionation Approach for Determination of Protein Precursors of Proteolytic Bioactive Metabolites from Macroalgae. J. Appl. Phycol. 2015, 27, 2059–2074. [Google Scholar] [CrossRef]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.E.; Turgeon, S.L. Characterization of Antibacterial Activity from Protein Hydrolysates of the Macroalga Saccharina Longicruris and Identification of Peptides Implied in Bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Hell, A.; Labrie, S.; Beaulieu, L. Effect of Seaweed Flakes Addition on the Development of Bioactivities in Functional Camembert-Type Cheese. Int. J. Food Sci. Technol. 2018, 53, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Rosemary, T.; Arulkumar, A.; Paramasivam, S.; Mondragon-Portocarrero, A.; Miranda, J.M. Biochemical, Micronutrient and Physicochemical Properties of the Dried Red Seaweeds Gracilaria Edulis and Gracilaria Corticata. Molecules 2019, 24, 2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown Macroalgae as Valuable Food Ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Escrig, A.B.; Sánchez-Muniz, F.J. Dietary Fiber from Edible Seaweeds: Chemical Struture, Physicochemical Properties and Effects on Cholesterol Metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohsugi, Y.; Yoshie, Y.; Shirai, T.; Hirano, T. Dietary Fiber Content, Water-Holding Capacity and Binding Capacity of Seaweeds. Fish. Sci. 1996, 62, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Lahaye, M.; Michel, C.; Luc Barry, J. Chemical, Physicochemical and in Vitro Fermentation Characteristics of Dietary Fibres from Palmaria palmata (L.) Kuntze. Food Chem. 1993, 47, 29–36. [Google Scholar] [CrossRef]
- Ramakrishnan, A.R.; Gana Sundari, G.; Mala, K.; Prakash, A. Preliminary Physico-Chemical Properties of Marine Macroalga Sargassum Tenerrimum (J. Agardh) (Fucales, Sargassaceae). Int. Res. J. Chem. 2015, 11, 21–43. [Google Scholar]
- Benjama, O.; Payap, M.; Masniyom, P. Nutritional Composition and Physicochemical Properties of Two Green Seaweeds (Ulva Pertusa and U. Intestinalis) from the Pattani Bay in Southern Thailand. Songklanakarin J. Sci. Technol. 2011, 33, 575–583. [Google Scholar]
- Chiu, T.H.; Chen, M.L.; Chang, H.C. Comparisons of Emulsifying Properties of Maillard Reaction Products Conjugated by Green, Red Seaweeds and Various Commercial Proteins. Food Hydrocoll. 2009, 23, 2270–2277. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary Fibre and Physicochemical Properties of Several Edible Seaweeds from the Northwestern Spanish Coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L. Seaweed carbohydrates. In Seaweed Sustainability: Food and Non-Food Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 141–192. ISBN 9780124199583. [Google Scholar]
- Ehrig, K.; Alban, S. Sulfated Galactofucan from the Brown Alga Saccharina Latissima-Variability of Yield, Structural Composition and Bioactivity. Mar. Drugs 2015, 13, 76–101. [Google Scholar] [CrossRef] [PubMed]
- Deniaud, E.; Quemener, B.; Fleurence, J.; Lahaye, M. Structural Studies of the Mix-Linked β-(1→3)/β-(1→4)-D-Xylans from the Cell Wall of Palmaria palmata (Rhodophyta). Int. J. Biol. Macromol. 2003, 33, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Harada, H.; Tsuchihashi, N.; Nagayama, S.; Nishide, E.; Innamii, S. Effect of Indigestible Polysaccharides on Pancreatic Exocrine Secretion and Biliary Output. J. Nutr. Sci. Vitaminol. 1984, 30, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, Y.; Sugase, K.; Horie, K. Physiological Differences of Soluble and Insoluble Dietary Fibre Fractions of Brown Algae and Mushrooms in Pepsin Activity in Vitro and Protein Digestibility. Asia Pac. J. Clin. Nutr. 1995, 4, 251–255. [Google Scholar] [PubMed]
- Maehre, H.K.; Edvinsen, G.K.; Eilertsen, K.E.; Elvevoll, E.O. Heat Treatment Increases the Protein Bioaccessibility in the Red Seaweed Dulse (Palmaria palmata), but Not in the Brown Seaweed Winged Kelp (Alaria Esculenta). J. Appl. Phycol. 2016, 28, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Marrion, O.; Schwertz, A.; Fleurence, J.; Guéant, J.L.; Villaume, C. Improvement of the Digestibility of the Proteins of the Red Alga Palmaria palmata by Physical Processes and Fermentation. Nahrung/Food 2003, 47, 339–344. [Google Scholar] [CrossRef]
- Marrion, O.; Fleurence, J.; Schwertz, A.; Guéant, J.L.; Mamelouk, L.; Ksouri, J.; Villaume, C. Evaluation of Protein in Vitro Digestibility of Palmaria palmata and Gracilaria Verrucosa. J. Appl. Phycol. 2005, 17, 99–102. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; de Boeck, G.; Becker, K. Dietary Roles of Non-Starch Polysachharides in Human Nutrition: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef]
- Fleury, N.; Lahaye, M. Chemical and Physico-chemical Characterisation of Fibres from Laminaria Digitata (Kombu Breton): A Physiological Approach. J. Sci. Food Agric. 1991, 55, 389–400. [Google Scholar] [CrossRef]
- Rupérez, P.; Toledano, G. Indigestible Fraction of Edible Marine Seaweeds. J. Sci. Food Agric. 2003, 83, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Denis, C.; Morancais, M.; Gaudin, P.; Fleurence, J. Effect of Enzymatic Digestion on Thallus Degradation and Extraction of Hydrosoluble Compounds from Grateloupia Turuturu. Bot. Mar. 2009, 52, 262–267. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Jaramillo-Torres, A.; Ahlstrøm, Ø.; Chikwati, E.; Aasen, I.M.; Kortner, T.M. Protein Value and Health Aspects of the Seaweeds Saccharina Latissima and Palmaria palmata Evaluated with Mink as Model for Monogastric Animals. Anim. Feed Sci. Technol. 2021, 276, 114902. [Google Scholar] [CrossRef]
- Sugita, D.; Joe, G.H.; Masuoka, M.; Konishi, Y.; Saeki, H. Effect of Drying Treatment on the Extractability and Anti-Inflammatory Function of Photosynthesis-Related Components in Dulse Palmaria palmata and Their Efficient Recovery from Dried Thallus. Fish. Sci. 2022, 88, 645–652. [Google Scholar] [CrossRef]
- Dupont, D.; Mackie, A.R. Static and Dynamic in Vitro Digestion Models to Study Protein Stability in the Gastrointestinal Tract. Drug Discov. Today Dis. Model. 2015, 17, 23–27. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; van de Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in Vitro Digestion Model in Assessing the Bioaccessibility of Mycotoxins from Food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Ordóñez, E.G. Evaluación Nutricional y Propiedades Biológicas de Algas Marinas Comestibles. Estudios “in Vitro” e “in Vivo”. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2013. [Google Scholar]
- Broch, O.J.; Slagstad, D. Modelling Seasonal Growth and Composition of the Kelp Saccharina Latissima. J. Appl. Phycol. 2012, 24, 759–776. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Ferrua, M.; Dalgleish, D.; Singh, H. Disintegration Kinetics of Food Gels during Gastric Digestion and Its Role on Gastric Emptying: An in Vitro Analysis. Food Funct. 2015, 6, 756–764. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. Modes of Disintegration of Solid Foods in Simulated Gastric Environment. Food Biophys. 2009, 4, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Bornhorst, G.M.; Ferrua, M.J.; Singh, R.P. A Proposed Food Breakdown Classification System to Predict Food Behavior during Gastric Digestion. J. Food Sci. 2015, 80, R924–R934. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.R.; Suarez-Garcia, E.; Safi, C.; Olivieri, G.; Olivieri, G.; Wijffels, R.H.; Wijffels, R.H. Energy Efficient Bead Milling of Microalgae: Effect of Bead Size on Disintegration and Release of Proteins and Carbohydrates. Bioresour. Technol. 2017, 224, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Maneesh, A.; Makkar, F. Antioxidant Activity of Brown Seaweeds. J. Aquat. Food Prod. Technol. 2017, 26, 406–419. [Google Scholar] [CrossRef]
- Suetsuna, K. Purification and Identification of Angiotensin I-Converting Enzyme Inhibitors from the Red Alga Porphyra Yezoensis. J. Mar. Biotechnol. 1998, 6, 163–167. [Google Scholar] [PubMed]
- Hata, Y.; Nakajima, K.; Uchida, J.-I.; Hidaka, H.; Nakano3, T. Clinical Effects of Brown Seaweed, Undaria Pinnatifida (Wakame), on Blood Pressure in Hypertensive Subjects. J. Clin. Biochem. Nutr. 2001, 30, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thorkelsson, G.; Ólafsdóttir, G. Enzyme-Enhanced Extraction of Antioxidant Ingredients from Red Algae Palmaria palmata. LWT 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Cian, R.E.; Caballero, M.S.; Sabbag, N.; González, R.J.; Drago, S.R. Bio-Accessibility of Bioactive Compounds (ACE Inhibitors and Antioxidants) from Extruded Maize Products Added with a Red Seaweed Porphyra Columbina. LWT 2014, 55, 51–58. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-Converting Enzyme (ACE) Inhibitory Activity of Fucus Spiralis Macroalgae and Influence of the Extracts Storage Temperature—A Short Report. J. Pharm. Biomed. Anal. 2016, 131, 503–507. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Horwitz, W., Ed.; Association of Official Analytical Chemists: Washington, WA, USA, 2002. [Google Scholar]
- Robertson, J.A. Physicochemical Characteristics of Food and the Digestion of Starch and Dietary Fibre during Gut Transit. Proc. Nutr. Soc. 1988, 47, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Oomen, A.G.; Rompelberg, C.J.M.; Bruil, M.A.; Dobbe, C.J.G.; Pereboom, D.P.K.H.; Sips, A.J.A.M. Development of an in Vitro Digestion Model for Estimating the Bioaccessibility of Soil Contaminants. Arch. Environ. Contam. Toxicol. 2003, 44, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Barbarino, E.; Lourenço, S.O. An Evaluation of Methods for Extraction and Quantification of Protein from Marine Macro- and Microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using O-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Ayala-Bribiesca, E.; Lussier, M.; Chabot, D.; Turgeon, S.L.; Britten, M. Effect of Calcium Enrichment of Cheddar Cheese on Its Structure, in Vitro Digestion and Lipid Bioaccessibility. Int. Dairy J. 2016, 53, 1–9. [Google Scholar] [CrossRef]
- Hayakari, M.; Kondo, Y.; Izumi, H. A Rapid and Simple Spectrophotometric Assay of Angiotensin-Converting Enzyme. Anal. Biochem. 1978, 84, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Verdon, C.P.; Wu, A.H.; Wang, H.; Prior, R.L. Automated Assay of Oxygen Radical Absorbance Capacity with the COBAS FARA II. Clin. Chem. 1995, 41, 1738–1744. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and Validation of Oxygen Radical Absorbance Capacity Assay for Lipophilic Antioxidants Using Randomly Methylated β-Cyclodextrin as the Solubility Enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and Hydrophilic Antioxidant Capacities of Common Foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Holden, J.; Haytowitz, D.B.; Gebhardt, S.E.; Beecher, G.; Prior, R.L. Development of a Database for Total Antioxidant Capacity in Foods: A Preliminary Study. J. Food Compos. Anal. 2004, 17, 407–422. [Google Scholar] [CrossRef]






| Species | Harvesting/ Origin | Soluble Fiber (%) | Insoluble Fiber (%) | Protein (%) |
|---|---|---|---|---|
| P. palmata | Wild | 10.52 ± 0.05 a | 12.19 ± 0.05 d | 19.87 ± 0.85 a |
| P. palmata | Cultivated | 10.61 ± 0.15 a | 18.81 ± 0.08 b | 19.31 ± 0.62 a |
| S. latissima | Wild | 5.91 ± 0.64 b | 32.44 ± 0.12 a | 16.97 ± 0.20 b |
| S. latissima | Cultivated | 3.77 ± 0.09 c | 13.92 ± 0.02 c | 15.03 ± 1.23 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, M.M.M.; Marson, G.V.; Rioux, L.-E.; Tamigneaux, E.; Turgeon, S.L.; Beaulieu, L. In Vitro Bioaccessibility of Proteins and Bioactive Compounds of Wild and Cultivated Seaweeds from the Gulf of Saint Lawrence. Mar. Drugs 2023, 21, 102. https://doi.org/10.3390/md21020102
Vasconcelos MMM, Marson GV, Rioux L-E, Tamigneaux E, Turgeon SL, Beaulieu L. In Vitro Bioaccessibility of Proteins and Bioactive Compounds of Wild and Cultivated Seaweeds from the Gulf of Saint Lawrence. Marine Drugs. 2023; 21(2):102. https://doi.org/10.3390/md21020102
Chicago/Turabian StyleVasconcelos, Margarida M. M., Gabriela V. Marson, Laurie-Eve Rioux, Eric Tamigneaux, Sylvie L. Turgeon, and Lucie Beaulieu. 2023. "In Vitro Bioaccessibility of Proteins and Bioactive Compounds of Wild and Cultivated Seaweeds from the Gulf of Saint Lawrence" Marine Drugs 21, no. 2: 102. https://doi.org/10.3390/md21020102