2.1. Structure Elucidation of Glycosides
The crude glycosidic sum of
Cucumaria djakonovi (1.379 g) was obtained after the hydrophobic chromatography of the concentrated ethanolic extract on a Polychrom-1 column (powdered Teflon, Biolar, Latvia). Then, it was chromatographed on Si gel columns (CC) with the stepped gradient of the eluents system of CHCl3/EtOH/H2O, in ratios of 100:50:4, 100:75:10, 100:100:17, and 100:125:25, and five fractions were obtained. The fractions 3−5, isolated after the repeated CC with the system of eluents CHCl
3/EtOH/H
2O (100:75:10), (100:100:17), and (100:125:25), were subsequently submitted to HPLC on reversed-phase semipreparative columns, Phenomenex Synergi Fusion RP (10 × 250 mm) and Synergi Hydro RP (10 × 250 mm), as well as chiral analytical column Kromasil 3-Cellucoat RP (4.6 × 150 mm), to give 10 individual novel and known glycosides (
Figure 1).
The sugar configurations in glycosides 1–4 were assigned as D on the basis of an analogy with all other known triterpene glycosides from sea cucumbers.
The molecular formula of djakonovioside C
1 (
1) was determined to be C
60H
93O
30SNa from the [M
Na – Na]
− ion peak at
m/
z 1325.5451 (calc. 1325.5478) in the (
−)HR-ESI-MS (
Figure S8). The structures of the identical aglycone moieties of djakonoviosides C
1 (
1) and E
1 (
3) and okhotoside A
2-1 (
5) were established by the analysis of their NMR spectra (
Table 1, (
Tables S1 and S2; Figures S1–S6, S17–S22, and S33–S38). The holostane-type aglycone (characteristic signals of 18(20)-lactone were observed at δ
C 180.2 (C-18) and 85.5 (C-20)) contains 7(8)- (the signals of CH-7 at δ
C 120.2 and δ
H 5.57 (m) and C-8 at δ
C 145.5) and 25(26)-double bonds (the signals of C-25 at δ
C 145.4 and CH
2-26 at δ
C 110.8 and δ
H 4.72 (m)) and 16β-acetoxy group (the signals at δ
C 75.3 (C-16), 170.7 (O
COCH
3), and 21.2 (OCO
CH
3). The orientation of this substituent was confirmed by the NOE correlation between H-16 and H-32 as well as by the value of the coupling constant (
J16/17 = 8.7 Hz) [
16].
Extensive analysis of the
1H,
13C NMR, and HSQC spectra of the carbohydrate moiety of djakonovioside C
1 (
1) (
Table 2;
Figures S1–S7) indicated the presence of the pentasaccharide chain with β-glycosidic bonds because five doublets of the anomeric protons at δ
H 4.73–5.25 (
J = 6.8–8.0 Hz) and the signals of the corresponding anomeric carbons at δ
C 102.4–105.6 were observed. Analysis of the
1H,
1H COSY, and 1D TOCSY spectra of each monosaccharide residue started from the anomeric proton, followed by ROESY and HSQC correlations analyses, allowed to determine the monosaccharide composition and the positions of glycosidic bonds. Hence, it was found that the second sugar in the chain is the xylose residue (Xyl2) that is linked to the Xyl1 residue at C-2. The second sugar unit (Xyl2) is bound with another two monosaccharides, being a branchpoint of the chain. The third monosaccharide unit—Glc3—was attached to C-4 Xyl2, and the additional xylose residue (Xyl5) was attached to C-2 Xyl2, causing glycosylation effects (δ
C-4 Xyl2 77.9 and δ
C-2 Xyl2 82.6). The analysis of the NMR spectra showed that 3-
O-methylglucose (MeGlc4) was a terminal monosaccharide unit linked to C-3 Glc3. The positions of the glycosidic linkages were corroborated by the ROESY and HMBC correlations between the H-1 Xyl1 and H-3 (C-3) of the aglycone, H-1 Xyl2 and H-2 (C-2) Xyl1, H-1 Glc3 and H-4 (C-4) Xyl2, H-1 MeGlc4 and H-3 (C-3) Glc3, and H-1 Xyl5 and H-2 (C-2) Xyl2 (
Table 2). The single sulfate group was attached to the C-4 Xyl1, causing an α-shifting effect of its signal to δ
C 76.1, instead of the δ
C ~70 observed in non-sulfated compounds.
The (
−)ESI-MS/MS of
1 (
Figure S8) demonstrated the fragmentation of the [M
Na − Na]
− ion, with
m/
z 1325.5 giving fragment ion peaks at
m/
z 1265.5 [M
Na − Na − CH
3COOH]
−, 1223.5 [M
Na − Na − NaSO
3 + H]
−, 1193.5 [M
Na − Na − Xyl]
−, 987.4 [M
Na − Na − MeGlc – Glc + H]
−, 813.2 [M
Na − Na – Agl − H]
−, 681.1 [M
Na − Na – Agl − Xyl]
−, and 595.2 [M
Na − Na − Agl − XylSO
3]
−. The (
+)ESI-MS/MS of
1 demonstrated the fragmentation of the [M
Na + Na]
+ ion, with
m/
z 1371.5 leading to ion peaks with
m/
z 1251.6 [M
Na + Na − NaHSO
4]
+ and 1179.6 [M
Na + Na − MeGlc + H]
+.
These data indicate that djakonovioside C1 (1) is 3β-O-{3-O-methyl-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→4)-[(1→2)-β-D-xylopyranosyl]-β-D-xylopyranosyl-(1→2)-4-O-sodium sulfate-β-D-xylopyranosyl}-16β-acetoxyholosta-7,25-dien.
The molecular formula of djakonovioside D
1 (
2) was determined as C
61H
95O
31SNa from the [M
Na − Na]
− ion peak at
m/
z 1355.5596 (calc. 1355.5584) and the [M
Na − Na − H]
2− ion peak at
m/
z 677.2767 (calc. 677.2755) in the (
−)HR-ESI-MS (
Figure S16). The aglycone of djakonovioside D
1 (
2) (
Table 3,
Figures S9–S15) was structurally close to that of
1, only differing by the position of the double bond in the side chain. So, all the signals of the polycyclic nuclei in the NMR spectra of
2 and
1 were almost coincident. The signals of the side chain were assigned by the analysis of the NMR spectra of
2: an isolated spin system formed by the protons from H-22 to H-24 was found in the
1H,
1H COSY spectrum, and the characteristic signals in the
13C and
1H NMR spectra at δ
C 123.9 (C-24), δ
H 5.00 (m, H-24) and 132.1 (C-25) corresponded to the 24(25)-double bond. Its position was confirmed by the correlations H-26(27)/C: 24, 25, 27(26) in the HMBC spectrum.
Extensive analysis of the
1H,
13C NMR,
1H,
1H COSY, 1D TOCSY, HSQC, and ROESY spectra of the carbohydrate part of djakonovioside D
1 (
2) and okhotoside A
2-1 (
5) (
Table 4 and
Table S3; Figures S9–S15 and S33–S38) revealed the presence of the same monosulphated pentasaccharide chains. The typical signals for quinovose residue were absent, while three signals characteristic for the hydroxymethylene groups of glucopyranose residues at δ
C 61.4, 61.1, and 61.7 were observed. Further analysis of the sugar composition and sequence, as well as the glycosidic bond positions, showed that the second and third units in the chain were glucose residues (Glc2 and Glc3); the 3-
O-methylglucose (MeGlc4) and xylose (Xyl5) residues were terminal, forming a carbohydrate chain branched by C-2 Glc2. A sulphate group was attached to C-4 Xyl1 (δ
C 76.1), as in djakonovioside C
1 (
1).
The fragment ion peaks in the (
−)ESI-MS/MS of
2 (
Figure S16) were observed at
m/
z 1296.5 [M
Na − Na − CH
3COOH]
−, 1105.5 [M
Na − Na − SO
3Na − Xyl + H]
−, 843.2 [M
Na − Na – Agl − H]
−, and 723.3 [M
Na − Na – Agl − NaSO
4]
− due to fragmentation of the [M
Na − Na]
− ion with
m/
z 1355.6. The ion peak at
m/
z 589.2 [M
Na − Na − MeGlc]
2− appeared as a result of the fragmentation of the [M
Na − Na − H]
2− ion at
m/
z 677.3. The (
+)ESI-MS/MS of
2 demonstrated the fragmentation of the [M
Na + Na]
+ ion with
m/
z 1401.5, giving ion peaks at
m/
z 1281.6 [M
Na + Na − NaHSO
4]
+ and 1209.6 [M
Na + Na − MeGlc + H]
+.
Thus, djakonovioside D1 (2) is 3β-O-{3-O-methyl-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→4)-[(1→2)-β-D-xylopyranosyl]-β-D-glucopyranosyl-(1→2)-4-O-sodium sulfate-β-D-xylopyranosyl}-16β-acetoxyholosta-7,24-dien.
The molecular formula of djakonovioside E
1 (
3) was determined as C
56H
85O
33S
3Na
3 from the [M
3Na − Na]
− ion peak at
m/
z 1427.3942 (calc. 1427.3936), the [M
3Na − 2Na]
2− ion peak at
m/
z 702.2037 (calc. 702.2022), and the three-charged ion [M
3Na − 3Na]
3− at
m/
z 460.4732 (calc. 460.4717) in the (
−)HR-ESI-MS (
Figure S24) that confirmed the presence of three sulfate groups. In the
1H and the
13C NMR spectra of the carbohydrate chain of djakonovioside E
1 (
3) (
Table 5,
Figures S17–S23), the four doublets of the anomeric protons at δ
H 4.68–5.09 (
J = 7.3–8.5 Hz) and the signals of the corresponding anomeric carbons at δ
C 103.8–104.7 were indicative of a tetrasaccharide chain with β-glycosidic bonds between sugars. The first monosaccharide connected to the C-3 of the aglycone was a xylose (Xyl1) sulfated by C-4 (deduced from the deshielding of this signal to δ
C 76.1). The subsequent analysis of the 1D TOCSY,
1H,
1H COSY, HSQC, and ROESY spectra revealed that the second residue was a glucose, which was linked to C-2 Xyl1. The third sugar—glucose (Glc3)—was attached to the typical position—C-4 Glc2 (cross-peak H-1 Glc3/H-4 Glc2 in the ROESY spectrum)—and was sulphated by C-6 (deshielding of the signal of C-6 Glc3 to δ
C 67.4). Terminal 3-
O-methylglucose residue was bound to C-3 Glc3, that was deduced from the corresponding NOE correlation (
Table 5) and also contained a sulphate group because the signal of C-6 MeGlc4 was observed at δ
C 67.0. Hence, the tetrasaccharide chain of
3 was new, having glucose as the second unit and bearing three sulfate groups.
The fragment ion peaks in the (
−)ESI-MS/MS of
3 (
Figure S24) were observed as a result of the fragmentation of the [M
3Na − Na]
− ion at
m/
z 1427.5, giving ions at
m/
z 1367.4 [M
3Na − Na − CH
3COOH]
−, 1307.4 [M
3Na − Na − NaHSO
4]
−, 1029.4 [M
3Na − Na − NaHSO
4–MeGlcSO
3 + H]
−, 915.1 [M
3Na − Na − Agl − H]
−, 681.1 [M
3Na − Na − Agl − XylSO
3 − H]
−, and 519.0 [M
3Na − Na − Agl − XylSO
3 − Glc − H]
−. The ion peak at
m/
z 446.0 [M
3Na − 2Na − Agl − H]
2− appeared as a result of the fragmentation of the [M
3Na − 2Na]
2− ion at
m/
z 704.2.
Thus, djakonovioside E1 (3) is 3β-O-[6-O-sodium sulfate-3-O-methyl-β-D-glucopyranosyl-(1→3)-6-O-sodium sulfate-β-D-glucopyranosyl-(1→4)-β-D-glucopyranosyl-(1→2)-4-O-sodium sulfate-β-D-xylopyranosyl]-16β-acetoxyholosta-7,25-dien.
The molecular formula of djakonovioside F
1 (
4) was determined as C
59H
93O
34S
3Na
3 from the [M
3Na − Na]
− ion peak at
m/
z 1487.4467 (calc. 1487.4511), the [M
3Na − 2Na]
2− ion peak at
m/
z 732.2320 (calc. 732.2310), and the [M
3Na − 3Na]
3− ion peak at
m/
z 480.4926 (calc. 480.4909) in the (−)HR-ESI-MS (
Figure S32). Noticeably, for the isotope composition of the pseudomolecular ion of
4, the predominance of the [M
3Na − Na + 2]
− ion peak is inherent. A normal isotope distribution was observed for two- and three-charged pseudomolecular ions. This is obviously explained by the chemical structure of the side chain. The protons of the methylene group CH
2-23 adjacent to the 22-oxo group and the 24(25)-double bond were easily exchanged to deuterium when the glycoside was dissolved in the mixture C
5D
5N/D
2O for the NMR spectra acquisition. The signal of CH
2-23 could not be accumulated in the
13C NMR spectrum of
4 for the same reason. Therefore, the spectra were repeatedly acquired in C
5D
5N/H
2O, which resulted in the appearance of the signal at δ
C 37.0 that corresponded to the proton’s signal at δ
H 3.61 (m, H-23) in the HSQC spectrum.
The aglycone of djakonovioside F
1 (
4) (
Table 6;
Figures S25–S30) did not contain a γ-lactone ring (deduced from the absence of the characteristic signal in the
13C NMR spectrum at δ
C ~180 (C-18)) being of the non-holostane type. Actually, the signals of CH
3-18 were observed at δ
C 24.5 and δ
H 1.29 (s). The signals in the downfield region of the
13C and
1H NMR spectra at δ
C 122.1 (C-7), δ
H 5.61 (m, H-7), and 148.5 (C-8) were characteristic of the intranuclear double bond, while the signals at δ
C 117.3 (C-24), δ
H 5.46 (m, H-24), and δ
C 134.9 (C-25) indicated the availability of a normal side chain with the 24(25)-double bond in
4. The signals of the side chain were deduced starting from the signal for C-22. The signal of C-22 was assigned on the basis of HMBC correlations of H-21/C: 20, 22 those are typical for lanostane derivatives. Hence, the signal of quaternary carbon C-22 was observed at δ
C 216.1, indicating the presence of an oxo-group. The position of the double bond was assigned as 24(25) based on the HMBC correlations of H-26(27)/C: 24, 25. The (20
R)-configuration, the same as in lanostane derivatives from sea cucumbers having an oxygen-containing substituent at C-22 [
17], was determined on the basis of NOE-correlations H-21/H-12 and H-21/H-17.
The
1H and
13C NMR spectra of the carbohydrate part of djakonovioside F
1 (
4) (
Table 7;
Figures S25–S31) were characteristic for the pentasaccharide chain (five doublets of anomeric protons at δ
H 4.71–5.20 and corresponding to the signals of the anomeric carbons at δ
C 102.1–105.2) with β-glycosidic bonds (coupling constants of anomeric protons
J = 7.2–8.1 Hz). The monosaccharide composition deduced from the analysis of the 1D TOCSY,
1H,
1H COSY, HSQC, and ROESY spectra was two xylose (Xyl1 and Xyl5), quinovose (Qui2), glucose (Glc3), and 3-
O-methylglucose (MeGlc4) residues. Analysis of the ROESY and HMBC spectra of
4 revealed that the quinovose was a branchpoint of the chain because Xyl5 attached to C-2 Qui2. The rest of the glycosidic bonds occupied typical positions for the glycosides of the sea cucumbers, demonstrating the glycosylation effects in the NMR spectra: δ
C-2 Xyl1 81.5, δ
C-4 Qui2 86.4, and δ
C-3 Glc3 86.6. Three sulphate groups were present in the sugar chain of
4 in the following positions, which were deduced on the basis of α-shifting effects: C-4 Xyl1 (δ
C 76.1), C-6Glc3 (δ
C 67.3), and C-6 MeGlc4 (δ
C 66.4). The same carbohydrate chain composed the molecules of isokoreoside A (
9) (
Table S7; Figures S52–S57) and koreoside A (
10) (
Table S9).
The fragment ion peaks in the (
−)ESI-MS/MS of
4 (
Figure S32) were observed as a result of the fragmentation of the [M
3Na − Na + 2]
− isotopic ion at
m/
z 1489.5, giving the ions at
m/
z 1211.4 [M
3Na − Na + 2−MeGlcSO
3]
−, 947.4 [M
3Na − Na + 2 − MeGlcSO
3 − GlcSO
3]
−, and 797.1 [M
3Na − Na + 2 − MeGlcSO
3 − GlcSO
3 − XylSO
3 − H]
−, arising as a result of the sequential loss of monosaccharide units.
Thus, djakonovioside F1 (4) is 3β-O-{6-O-sodium sulfate-3-O-methyl-β-D-glucopyranosyl-(1→3)-6-O-sodium sulfate-β-D-glucopyranosyl-(1→4)-[(1→2)-β-D-xylopyranosyl]-β-D-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-D-xylopyranosyl}-(20R)-hydroxy-22-oxo-lanosta-7,24-dien.
The structures of known compounds, okhotoside A
2-1 (
5) from
C. okhotensis [
18], cucumarioside A
2-5 (
6) from
C. conicospermium [
19], frondoside A
2-3 (
7) from
C. frondosa [
20], and koreoside A (
10) from C.
koreaensis [
21], were identified by extensive analysis of the 1D and 2D NMR spectra and compared with the literature data. All the original spectra and the assignments of the signals are provided in
Tables S2–S9 and Figures S33–S57.
Isokoreoside A (
9) (
Tables S7–S8; Figures S52–S57) was first isolated as a desulfated derivative from the fraction of the trisulfated glycosides of
C. conicospermium [
19], which was separated into individual compounds after the procedure of solvolytic desulfation. In native form, this compound was later obtained from
C. frondosa [
17].
Cucumarioside A
3-2 (
8) was isolated as native glycoside from
C. djakonovi for the first time. The structure of
8 was determined earlier on the basis of its desulfated derivative, obtained the same way as
9 from
Cucumaria conicospermium [
19]. So, the 2D NMR spectra and the assignments of the signals of
8 are provided first (
Table 8 and
Table 9,
Figures S45–S51).
2.2. Biologic Activity of the Glycosides
The cytotoxic activity of the compounds isolated from
C. djakonovi was studied against three types of human breast cancer cells (MCF-7, T-47D, and triple negative MDA-MB-231), as well as the non-tumor mammary epithelial cell line MCF-10A. Djakonovioside A
1 [
5] and cisplatin were used as the positive controls. Cytotoxic activity against all the selected cell lines was assessed using the MTT method (
Table 10).
Djakonovioside F1 (4), okhotoside A2-1 (5), and cucumarioside A2-5 (6) showed strong hemolytic activity against human erythrocytes, with ED50 0.51 ± 0.01, 1.53 ± 0.14, and 1.63 ± 0.13 µM, respectively. The hemolytic activity of djakonoviosides C1 (1) and D1 (2) was slightly lower but significant and close to each other. Djakonovioside D1 (2), cucumarioside A3-2 (8), and isokoreoside A (9) demonstrated moderate hemolytic activity but were not active against all human tumor cell lines. Frondoside A2-3 (7) and koreoside A (10) did not show hemolytic or cytotoxic activity in the concentration range up to 50 µM.
The estimation of the selectivity index (
Table 11) showed djakonovioside E
1 (
3) was a leader, demonstrating the strongest cytotoxicity against the MCF-7 cell line (IC
50 1.52 ± 0.14 μM) as well as against the triple negative MDA-MB-231 cell line (IC
50 2.19 ± 0.17 µM). At the same time, this glycoside was not toxic in relation to normal mammary epithelial cells (MCF-10A). None of the other glycosides showed similar selectivity. But the MDA-MB-231 cell line was more sensitive to their action compared with the T-47D and, especially, MCF-7 cell lines.
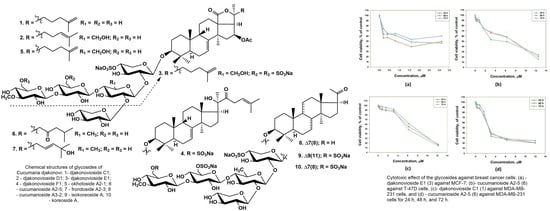
The cytotoxic activity of djakonovioside E
1 (
3) was maximal in the series against the MCF-7 and MDA-MB-231 cell lines with a half-maximal inhibitory concentration of 1.52 ± 0.14 μM (
Figure 2a) and 2.19 ± 0.17 µM (
Table 10), respectively. Cucumarioside A
2-5 (
6) was the most active compound from the series in relation to T-47D cells (IC
50 5.81 ± 0.86 μM (
Figure 2b). Djakonovioside C
1 (
1) and cucumarioside A
2-5 (
6) demonstrated a pronounced effects against the MDA-MB-231 cell line (IC
50 of 7.67 ± 0.32 and 2.58 ± 0.1 μM, respectively) (
Figure 2c,d).
To study antiproliferative properties, three most active glycosides were selected: djakonoviosides C
1 (
1) and E
1 (
3) and cucumarioside A
2-5 (
6). The prolonged incubation of cells for 48 and 72 h with glycosides
1 and
6 did not increase their EC
50; but, more importantly, the glycosides did not lose cytotoxicity over time. Only djakonovioside E
1 (
3) showed an antiproliferative effect when incubated with MCF-7 cells for 48 h, demonstrating approximately a two-fold increase in EC
50 (0.78 ± 0.32 μM) (
Figure 2a).
The clonogenic (or colony formation) assay is a standard in vitro cell survival assay based on the ability of a single cell to grow into a colony. In the human body, this uncontrolled growth of tumor cells leads to the formation of metastases. To study the effect of selected glycosides on the formation and growth of tumor cell colonies, a range of non-toxic concentrations was used against the MDA-MB-231 cell line (
Figure 3a). Additionally, colonies of MCF-7 lines were exposed to the action of non-toxic doses of djakonovioside E
1 (
3) (
Figure 3b). For all tested compounds, a dose-dependent effect of inhibiting colony growth was observed. Cucumarioside A
2-5 (
6) at a concentration of 1 μM demonstrated the greatest inhibitory effect on the formation and growth of colonies: 44.32 ± 0.77% of the control. The inhibitory effects of djakonovioside C
1 (
1) at concentrations of 2 and 1 µM were the same—approximately 25% compared to the control. Djakonovioside E
1 (
3) blocked the formation of colonies of both MDA-MB-231 and MCF-7 to the same extent: at a concentration of 2 μM in relation to MDA-MB-231 cells to 35.68 ± 2.00% of the control and in relation to MCF-7 cells to 30.73 ± 0.49% of the control (
Figure 3a,b).
Scratch analysis is used to study the effects of compounds with potential antitumor activity on cell motility and cell–cell interactions. In the control group of the MDA-MB-231 cell line, the scratch was overgrown within 24 h, while in the control group of the MCF-7 cell line, this happened after 72 h (
Figure 4e). All the selected glycosides in the concentration range below their EC
50 inhibited the migration of breast cancer MDA-MB-231 and MCF-7 cells in a dose-dependent manner. The greatest effect, about 85% as compared to the control, was observed for cucumarioside A
2-5 (
6) at a concentration of 1 μM after 24 h of incubation with MDA-MB-231 cells (
Figure 4b). Images of CFDA SE fluorescently labeled MCF-7 cells incubated with djakonovioside E
1 (
3) demonstrate the reliable blockage of the migration of cells of the MCF-7 line under the glycoside action (
Figure 4e).
2.3. Correlational Analysis and QSAR Model
The quantitative structure–activity relationship (QSAR) approach was applied to analyze the correlation between the hemolytic activity values and structures of all the glycosides isolated from
C. djakonovi. Three-dimensional models of the glycosides were built, protonated at pH 7.4, and subjected to energy minimization. A conformational search was performed with MOE 2020.0901 CCG software [
22], and the dominant glycoside conformations were selected for further analysis. A set of various 2D and 3D descriptors (379 in total) responsible for physicochemical properties, as well as energy values and topological indexes numerically expressing the geometric properties of molecular structures, was calculated and analyzed using the QuaSAR-Descriptor tool of the MOE 2020.0901 CCG software [
22].
Noticeably, the descriptors choice has a fundamental significance, since no “almighty descriptor set” modeling all the activities and properties has been found yet. So, the selection of a suitable descriptor set for each activity and type of analyzed compounds is needed. So, in addition to the descriptors characterizing the physicochemical properties of the molecules (polarizability, refractive index, surface charge distribution, dipole moment, hydrogen bonds’ potential strength (donors and acceptors) [
23], hydrophobic volume, surface area, atomic valence connectivity index, etc.), following descriptors as the presence/absence of 18(20)-lactone and the side chain, carbohydrate chain branching, and nature of the second sugar residue (glucose, quinovose, and xylose), the sulfate groups’ number and positions were added to the descriptors set provided by the MOE-QuaSAR-Descriptor software (2020.0901 CCG). The correlational analysis revealed the direct positive correlation between the hemolytic activities of the tested compounds in vitro and such descriptors as the molecular refractivity, log of the octanol/water partition coefficient [
24], and partial charges distribution on the van der Waals surface area. In contrast, the diameter of the molecule, principal moment of inertia describing the different aspects of molecular shape, VDW surface area (Å
2), molecular VDW volume (Å
3), lowest hydrophobic energy, and approximation of the sum of the VDW surface areas of hydrophobic atoms (Å
2) were found to negatively correlate.
The analysis of the principal components (PCA) decreased the number of descriptors, leaving only those having a substantial contribution, and resulted in the division of the glycosides into two groups (
Figure 5), which indicated the right way of the descriptor’s choice. The linear QSAR model was built with the QuaSAR-Model tool of the MOE 2020.0901 CCG software [
22] using these descriptors. The model fits well with the experimental data on the hemolytic activities of glycosides with a correlation coefficient r
2 = 0.94702 and RMSE = 0.05234 (
Figure S58). The model was cross-validated with r
2cros = 0.82341 and RMSE
cros = 0.24613. The QSAR model includes 148 terms, 58 from those that have the biggest contribution; however, the reduction in the number of descriptors to the latter value resulted in the quality deterioration of the correlation model. All these data indicate the extremely complex nature of the relationships between the structure of glycosides and their membranolytic action, with the multiple negligible effects of plenty of descriptors causing a considerable effect in combination with each other.