Structural Characterization and Rheological and Antioxidant Properties of Novel Polysaccharide from Calcareous Red Seaweed
Abstract
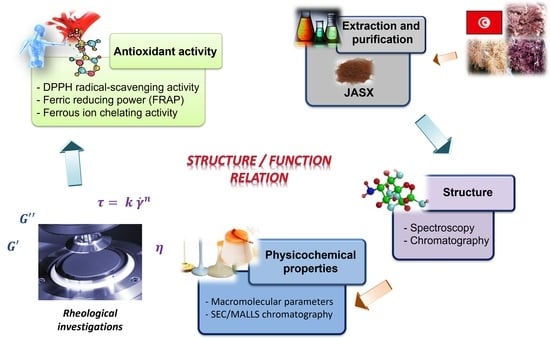
:1. Introduction
2. Results and Discussion
2.1. Structural Analysis of JASX
2.1.1. Extraction Yield and Biochemical Composition
2.1.2. Monosaccharide Analysis
2.1.3. ATR-FTIR Spectroscopy
2.1.4. NMR Spectroscopy
2.1.5. Molar Mass and Macromolecular Characteristics of JASX
2.2. Rheological Properties of JASX
2.2.1. Steady-Shear Flow Measurements of JASX
2.2.2. Dynamic Viscoelastic Properties of JASX
2.2.3. Critical Overlap Concentration (C*) of JASX
2.2.4. Thixotropic Properties of JASX
2.3. Antioxidant Activities of JASX
2.3.1. DPPH Radical-Scavenging Activity
2.3.2. Ferric-Reducing Power
2.3.3. Ferrous Ion-Chelating Activity
3. Material and Methods
3.1. Marine Seaweed Collection
3.2. Extraction and Purification of Jania adhaerens Sulfated Xylogalactan (JASX)
3.3. Global Biochemical Composition of JASX
3.4. Solvolytic Desulfation of Polysaccharide
3.5. Structural Features of JASX
3.5.1. ATR-FTIR Analysis
3.5.2. Determination of Monosaccharide Composition by GC/MS
3.5.3. SEC-MALLS Analysis of JASX
3.5.4. NMR Spectroscopy Analysis
3.6. Rheological Investigations
3.6.1. Rheological Measurements
3.6.2. Steady-Shear Flow Measurements
3.6.3. Dynamic Viscoelastic Properties
3.7. Antioxidant Activity
3.7.1. DPPH Radical-Scavenging Activity
3.7.2. Ferric-Reducing Power
3.7.3. Ferrous Ion-Chelating Power
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fenoradosoa, T.; Delattre, C.; Laroche, C.; Wadouachi, A.; Dulong, V.; Picton, L.; Andriamadio, P.; Michaud, P. Highly sulphated galactan from Halymenia durvillei (Halymeniales, Rhodophyta), a red seaweed of Madagascar marine coasts. Int. J. Biol. Macromol. 2009, 45, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Benaoun, F.; Delattre, C.; Boual, Z.; Ursu, A.V.; Vial, C.; Gardarin, C.; Wadouachi, A.; Le Cerf, D.; Varacavoudin, T.; El-Hadj, M.D.O.; et al. Structural characterization and rheological behavior of a heteroxylan extracted from Plantago notata Lagasca (Plantaginaceae) seeds. Carbohydr. Polym. 2017, 175, 96–104. [Google Scholar] [CrossRef]
- Barros, F.C.; da Silva, D.C.; Sombra, V.G.; Maciel, J.S.; Feitosa, J.P.; Freitas, A.L.; de Paula, R.C. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh). Carbohydr. Polym. 2013, 92, 598–603. [Google Scholar] [CrossRef]
- Lajili, S.; Ammar, H.H.; Mzoughi, Z.; Amor, H.B.H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Characterization of sulfated polysaccharide from Laurencia obtusa and its apoptotic, gastroprotective and antioxidant activities. Int. J. Biol. Macromol. 2018, 126, 326–336. [Google Scholar] [CrossRef]
- Bilan, M.I.; Usov, A.I. Polysaccharides of calcareous algae and their effect on calcification process. Russ. J. Bioorg. Chem. 2001, 27, 2–16. [Google Scholar] [CrossRef]
- Cases, M.R.; Stortz, C.A.; Cerezo, A.S. Structure of the ‘corallinans’—Sulfated xylogalactans from Corallina officinalis. Int. J. Biol. Macromol. 1994, 16, 93–97. [Google Scholar] [CrossRef]
- Takano, R.; Hayashi, J.; Hara, S.; Hirase, S. Structure of a Water-soluble Polysaccharide Sulfate from the Red Seaweed Joculator maximus Manza. Bot. Mar. 1996, 39, 95–102. [Google Scholar] [CrossRef]
- Navarro, D.A.; Stortz, C.A. Isolation of xylogalactans from the Corallinales: Influence of the extraction method on yields and compositions. Carbohyd. Polym. 2002, 49, 57–62. [Google Scholar] [CrossRef]
- Navarro, D.A.; Ricci, A.M.; Rodríguez, M.C.; Stortz, C.A. Xylogalactans from Lithothamnion heterocladum, a crustose member of the Corallinales (Rhodophyta). Carbohydr. Polym. 2011, 84, 944–951. [Google Scholar] [CrossRef]
- Navarro, D.A.; Stortz, C.A. The system of xylogalactans from the red seaweed Jania rubens (Corallinales, Rhodophyta). Carbohydr. Res. 2008, 343, 2613–2622. [Google Scholar] [CrossRef]
- Manghisi, A.; Miladi, R.; Minicante, S.A.; Genovese, G.; Le Gall, L.; Abdelkafi, S.; Saunders, G.W.; Morabito, M. DNA Barcoding Sheds Light on Novel Records in the Tunisian Red Algal Flora. Cryptogam. Algol. 2019, 40, 5–27. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Gardarin, C.; Desbrières, J.; Le Cerf, D.; Rihouey, C.; Michaud, P.; Abdelkafi, S.; Pierre, G. Structural Features and Rheological Properties of a Sulfated Xylogalactan-Rich Fraction Isolated from Tunisian Red Seaweed Jania adhaerens. Appl. Sci. 2020, 10, 1655. [Google Scholar] [CrossRef]
- Zeid, A.A.; Aboutabl, E.; Sleem, A.; El-Rafie, H. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carbohydr. Polym. 2014, 113, 62–66. [Google Scholar] [CrossRef]
- Maciel, J.S.; Chaves, L.S.; Souza, B.W.S.; Teixeira, D.I.; Freitas, A.L.; Feitosa, J.P.; de Paula, R. Structural characterization of cold extracted fraction of soluble sulfated polysaccharide from red seaweed Gracilaria birdiae. Carbohydr. Polym. 2008, 71, 559–565. [Google Scholar] [CrossRef]
- Chiovitti, A.; Bacic, A.; Craik, D.; Kraft, G.T.; Liao, M.L.; Falshaw, R.; Furneaux, R. A pyruvated carrageenan from Australian specimens of the red alga Sarconema filiforme. Carbohydr. Res. 1998, 310, 77–83. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Wu, J.; Chen, Y.; Wang, S. In vitro antioxidant activities of sulfated polysaccharide fractions extracted from Corallina officinalis. Int. J. Biol. Macromol. 2011, 49, 1031–1037. [Google Scholar] [CrossRef]
- Sudharsan, S.; Giji, S.; Seedevi, P.; Vairamani, S.; Shanmugam, A. Isolation, characterization and bioactive potential of sulfated galactans from Spyridia hypnoides (Bory) Papenfuss. Int. J. Biol. Macromol. 2018, 109, 589–597. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Sekkal, M.; Legrand, P. A spectroscopic investigation of the carrageenans and agar in the 1500-100 cm−1 spectral range. Spectrochim. Acta Part A Mol. Spectrosc. 1993, 49, 209–221. [Google Scholar] [CrossRef]
- Souza, B.W.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Shashkov, A.S. Structure of a sulfated xylogalactan from the calcareous red alga Corallina pilulifera P. et R. (Rhodophyta, Corallinaceae). Carbohydr. Res. 1997, 303, 93–102. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I. Polysaccharides of algae. 52. The structure of sulfated xylogalactan from the calcareous red alga bossiella cretacea (P. et R.) Johansen (rhodophyta, corallinaceae). Russ. J. Bioorganic Chem. 1998, 24, 123–129. [Google Scholar]
- Restrepo-Espinosa, D.C.; Román, Y.; Colorado-Ríos, J.; de Santana-Filho, A.P.; Sassaki, G.L.; Cipriani, T.R.; Martínez, A.; Iacomini, M.; Pavão, M.S. Structural analysis of a sulfated galactan from the tunic of the ascidian Microcosmus exasperatus and its inhibitory effect of the intrinsic coagulation pathway. Int. J. Biol. Macromol. 2017, 105, 1391–1400. [Google Scholar] [CrossRef]
- Murano, E.; Toffanin, R.; Zanetti, F.; Knutsen, S.; Paoletti, S.; Rizzo, R. Chemical and macromolecular characterisation of agar polymers from Gracilaria dura (C. Agardh) J. Agardh (Gracilariaceae, Rhodophyta). Carbohydr. Polym. 1992, 18, 171–178. [Google Scholar] [CrossRef]
- Pomin, V.H. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj. J. 2009, 27, 1–12. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.M.; Wang, X.F.; Liu, Z.Y.; Zhang, J.Y.; Guo, Y.J.; Chen, W.Z.; Liu, Y.; Cheong, K.L. Physicochemical characterization of Gracilaria chouae sulfated polysaccharides and their antioxidant potential. Int. J. Biol. Macromol. 2019, 134, 255–261. [Google Scholar] [CrossRef]
- Hentati, F.; Pierre, G.; Ursu, A.V.; Vial, C.; Delattre, C.; Abdelkafi, S.; Michaud, P. Rheological investigations of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Food Hydrocoll. 2020, 103, 105631. [Google Scholar] [CrossRef]
- Razmkhah, S.; Razavi, S.M.A.; Mohammadifar, M.A. Dilute solution, flow behavior, thixotropy and viscoelastic characterization of cress seed (Lepidium sativum) gum fractions. Food Hydrocoll. 2017, 63, 404–413. [Google Scholar] [CrossRef]
- Balaghi, S.; Mohammadifar, M.A.; Zargaraan, A.; Gavlighi, H.A.; Mohammadi, M. Compositional analysis and rheological characterization of gum tragacanth exudates from six species of Iranian Astragalus. Food Hydrocoll. 2011, 25, 1775–1784. [Google Scholar] [CrossRef]
- Yin, J.Y.; Wang, J.Q.; Lin, H.X.; Xie, M.Y.; Nie, S.P. Fractionation, physicochemical properties and structural features of non-arabinoxylan polysaccharide from the seeds of Plantago asiatica L. Food Hydrocoll. 2016, 55, 128–135. [Google Scholar] [CrossRef]
- Chouana, T.; Pierre, G.; Vial, C.; Gardarin, C.; Wadouachi, A.; Cailleu, D.; Le Cerf, D.; Boual, Z.; El Hadj, M.O.; Michaud, P.; et al. Structural characterization and rheological properties of a galactomannan from Astragalus gombo Bunge seeds harvested in Algerian Sahara. Carbohydr. Polym. 2017, 175, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef]
- Abad, L.V.; Relleve, L.S.; Racadio, C.D.T.; Aranilla, C.T.; De la Rosa, A.M. Antioxidant activity potential of gamma irradiated carrageenan. Appl. Radiat. Isot. 2013, 79, 73–79. [Google Scholar] [CrossRef]
- Qu, G.; Liu, X.; Wang, D.; Yuan, Y.; Han, L. Isolation and characterization of fucoidans from five brown algae and evaluation of their antioxidant activity. J. Ocean Univ. China 2014, 13, 851–856. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Vancamp, J.; Demeulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Alves, M.G.C.F.; Dore, C.M.P.G.; Castro, A.J.G.; Nascimento, M.S.D.; Cruz, A.K.M.; Soriano, E.M.; Benevides, N.M.B.; Leite, E.L. Antioxidant, cytotoxic and hemolytic effects of sulfated galactans from edible red alga Hypnea musciformis. J. Appl. Phycol. 2011, 24, 1217–1227. [Google Scholar] [CrossRef]
- Alencar, P.O.C.; Lima, G.C.; Barros, F.C.N.; Costa, L.E.; Ribeiro, C.V.P.; Sousa, W.M.; Sombra, V.G.; Abreu, C.M.W.; Abreu, E.S.; Pontes, E.O.; et al. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In vitro and in vivo activities. Food Hydrocoll. 2018, 90, 28–34. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.C. Colorimetric determination of neutral sugars by a resorcinol sulphuric acid micromethod. Anal. Chem. 1988, 175, 525–530. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Yaphe, W.; Arsenault, G. Improved resorcinol reagent for the determination of fructose, and of 3,6-anhydrogalactose in polysaccharides. Anal. Biochem. 1965, 13, 143–148. [Google Scholar] [CrossRef]
- Sloneker, J.H.; Orentas, D.G. Pyruvic Acid, a Unique Component of an Exocellular Bacterial Polysaccharide. Nature 1962, 194, 478–479. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of totalphenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Pierre, G.; Graber, M.; Rafiliposon, B.A.; Dupuy, C.; Orvain, F.; De Crignis, M.; Maugard, T. Biochemical Composition and Changes of Extracellular Polysaccharides (ECPS) Produced during Microphytobenthic Biofilm Development (Marennes-Oléron, France). Microb. Ecol. 2011, 63, 157–169. [Google Scholar] [CrossRef]
- Pierre, G.; Zhao, J.M.; Orvain, F.; Dupuy, C.; Klein, G.L.; Graber, M.; Maugard, T. Seasonal dynamics of extracellular polymeric substances (EPS) in surface sediments of a diatom-dominated intertidal mudflat (Marennes-Oléron, France). J. Sea Res. 2014, 92, 26–35. [Google Scholar] [CrossRef]
- Kirby, A.J.; Schmidt, R.J. The antioxidant activity of Chinese herbs for eczema and of placebo herbs—I. J. Ethnopharmacol. 1997, 56, 103–108. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of Antioxidant and Antimicrobial Activities of Rumex crispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]







| Extraction Yield (%, w/w) | Total Sugar (% w/w) | Neutral Sugar (% w/w) | Uronic Acid (% w/w) | Proteins (% w/w) | Phenolic Compounds (% w/w) | Sulfate (% w/w) | Pyruvate (% w/w) | 3,6-AnGal (% w/w) | NaCl (%) |
|---|---|---|---|---|---|---|---|---|---|
| 5.25 | 73.52 ± 0.85 | 68.02 ± 0.76 | 6.55 ± 0.61 | 0.64 ± 0.06 | 0.66 ± 0.02 | 12.55 ± 0.61 | 0.35 ± 0.04 | 19.53 ± 1.16 | 1.25 |
| Monosaccharide Composition (Molar %) a | Mw b (kDa) | Mn c (kDa) | Đ d | Rh e (nm) | [η] f (mL.g−1) | C* g (g.L−1) | |||
|---|---|---|---|---|---|---|---|---|---|
| Galp | Xylp | Glcp | GlcpA | ||||||
| 73.06 | 16.66 | 8.46 | 1.81 | 600 | 160 | 3.7 | 17.2 | 102 | 7.0 |
| JASX (%, w/v) | NaCl (mol.L−1) | n | k (Pa.sn) | R2 |
|---|---|---|---|---|
| 0.25 | 0.0 | 0.70 ± 0.014 | 0.018 ± 0.001 | 0.98 |
| 0.5 | 0.75 ± 0.022 | 0.015 ± 0.004 | 0.98 | |
| 0.50 | 0.0 | 0.66 ± 0.009 | 0.038 ± 0.002 | 0.99 |
| 0.5 | 0.69 ± 0.013 | 0.032 ± 0.001 | 0.98 | |
| 0.75 | 0.0 | 0.59 ± 0.015 | 0.118 ± 0.009 | 0.99 |
| 0.5 | 0.64 ± 0.032 | 0.103 ± 0.005 | 0.97 | |
| 1.0 | 0.0 | 0.56 ± 0.024 | 0.187 ± 0.012 | 0.99 |
| 0.5 | 0.60 ± 0.019 | 0.162 ± 0.008 | 0.98 | |
| 1.5 | 0.0 | 0.53 ± 0.005 | 0.417 ± 0.023 | 0.99 |
| 0.5 | 0.57 ± 0.017 | 0.399 ± 0.018 | 0.99 | |
| 2.0 | 0.0 | 0.52 ± 0.021 | 0.871 ± 0.044 | 0.99 |
| 0.5 | 0.55 ± 0.034 | 0.852 ± 0.032 | 0.98 |
| JASX (%, w/v) | Parameters | Shear Rate (s−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3.981 | 10 | 39.81 | 100 | 398.1 | 1000 | ||
| 1.0 | Ea (Kcal.mol−1) | 5.50 | 5.10 | 5.03 | 4.72 | 4.63 | 4.20 | 4.02 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |
| 1.5 | Ea (Kcal.mol−1) | 5.49 | 5.11 | 5.03 | 4.70 | 4.63 | 4.17 | 3.99 |
| R2 | 0.99 | 0.99 | 0.99 | 1.00 | 0.99 | 0.99 | 0.99 | |
| 2.0 | Ea (Kcal.mol−1) | 5.47 | 5.02 | 4.93 | 4.67 | 4.58 | 4.13 | 3.92 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hentati, F.; Tounsi, L.; Pierre, G.; Barkallah, M.; Ursu, A.V.; Ben Hlima, H.; Desbrières, J.; Le Cerf, D.; Fendri, I.; Michaud, P.; et al. Structural Characterization and Rheological and Antioxidant Properties of Novel Polysaccharide from Calcareous Red Seaweed. Mar. Drugs 2022, 20, 546. https://doi.org/10.3390/md20090546
Hentati F, Tounsi L, Pierre G, Barkallah M, Ursu AV, Ben Hlima H, Desbrières J, Le Cerf D, Fendri I, Michaud P, et al. Structural Characterization and Rheological and Antioxidant Properties of Novel Polysaccharide from Calcareous Red Seaweed. Marine Drugs. 2022; 20(9):546. https://doi.org/10.3390/md20090546
Chicago/Turabian StyleHentati, Faiez, Latifa Tounsi, Guillaume Pierre, Mohamed Barkallah, Alina Violeta Ursu, Hajer Ben Hlima, Jacques Desbrières, Didier Le Cerf, Imen Fendri, Philippe Michaud, and et al. 2022. "Structural Characterization and Rheological and Antioxidant Properties of Novel Polysaccharide from Calcareous Red Seaweed" Marine Drugs 20, no. 9: 546. https://doi.org/10.3390/md20090546