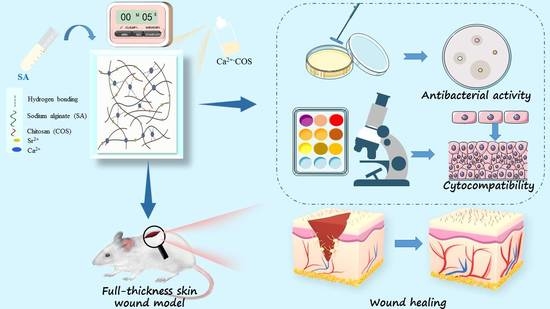
Preparation of Ion2+-COS/SA Multifunctional Gel Films for Skin Wound Healing by an In Situ Spray Method
Abstract
:1. Introduction
2. Results and Discussions
2.1. Preparation Mechanism of COS/SA/M2+ PDF
2.2. Chemical Structure and Property Analysis of COS/SA/M2+ PDF
2.2.1. Surface Appearance
2.2.2. FTIR
2.2.3. XRD
2.2.4. Contact Angle
2.2.5. Inflationary Behavior
2.2.6. Porosity
2.2.7. Thermal Stability
2.3. Mechanical and Rheological Analysis of COS/SA/M2+ PDF
2.4. Antioxidant Activity of COS/SA/M2+ PDF
2.5. Antibacterial Properties of COS/SA/M2+ PDF
2.6. Cytocompatibility of COS/SA/M2+ PDF
2.7. In Vivo Healing Promotion Experiment of COS/SA/M2+ PDF
2.7.1. Wound Healing Assessment
2.7.2. Histological Evaluation of the Regenerated Tissue
2.7.3. Expression of FGF and CD31 during Wound Healing
3. Materials and Methods
3.1. Experimental Materials
3.2. Preparation of Ion2+-COS/SA Gel Films
3.3. SEM Morphological Analysis
3.4. FTIR
3.5. XRD
3.6. Contact Angle
3.7. Mechanical and Rheological Property Testing
3.8. Inflationary Behavior
3.9. Porosity Measurements
3.10. Thermal Stability
3.11. Antioxidant Activity Analysis
3.11.1. FRAP Analysis
3.11.2. DPPH Free Radical Scavenging Activity
3.11.3. Ability to Scavenge ABTS Free Radicals
3.12. Antibacterial Activity Analysis
3.13. Cell Viability Assay
3.14. In Vivo Healing Promotion Experiment
3.14.1. Construction of the Trauma Models
3.14.2. Histological Assessment
3.14.3. Immunohistochemistry Analysis
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, C.; McGrath, E. Outcomes in wound healing after lower leg skin surgery. Brit. J. Dermatol. 2021, 185, 115–116. [Google Scholar]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eming, S.A.; Koch, M.; Krieger, A.; Brachvogel, B.; Kreft, S.; Bruckner-Tuderman, L.; Krieg, T.; Shannon, J.D.; Fox, J.W. Differential Proteomic Analysis Distinguishes Tissue Repair Biomarker Signatures in Wound Exudates Obtained from Normal Healing and Chronic Wounds. J. Proteome Res. 2010, 9, 4758–4766. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.H.; Molavi, B.; Mohammadi, S.; Nikbakht, M.; Mohammadi, A.M.; Mostafaei, S.; Norooznezhad, A.H.; Abdegah, A.G.; Ghavamzadeh, A. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A single-arm clinical trial. Transfus. Apher. Sci. 2017, 56, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Paolini, G.; Sorotos, M.; Firmani, G.; Gravili, G.; Ceci, D.; di Pompeo, F.S. Low-vacuum negative pressure wound therapy protocol for complex wounds with exposed vessels. J. Wound Care 2022, 31, 78–85. [Google Scholar] [CrossRef]
- Matsumae, G.; Terkawi, M.A.; Nonoyama, T.; Kurokawa, T.; Takahashi, D.; Shimizu, T.; Kadoya, K.; Gong, J.P.; Yasuda, K.; Iwasaki, N. Evaluation of biological responses to micro-particles derived from a double network hydrogel. Biomater. Sci. 2022, 10, 2182–2187. [Google Scholar] [CrossRef]
- Li, S.N.; Li, B.Q.; Gong, L.X.; Tang, L.C.; Feng, Y.J.; Jia, D.C.; Zhou, Y. Hyperbranched polysiloxane for highly stretchable and tough hydrogel by one-pot in situ polymerization. J. Control. Release 2017, 259, E122. [Google Scholar] [CrossRef]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef]
- Lin, Z.K.; Yang, Y.R.; Liang, Z.Z.; Zeng, L.; Zhang, A.P. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.Y.; Kang, Y.; Zeng, X.T.; Xie, Y.; Zhang, Y.K.; Wang, Y.B.; Xie, T.H. Preparation of temperature-sensitive Xanthan/NIPA hydrogel using citric acid as crosslinking agent for bisphenol A adsorption. Carbohyd. Polym. 2019, 206, 94–101. [Google Scholar] [CrossRef]
- He, G.; Lei, H.; Sun, W.; Gu, J.; Yu, W.; Zhang, D.; Chen, H.; Li, Y.; Qin, M.; Xue, B.; et al. Strong and Reversible Covalent Double Network Hydrogel Based on Force-Coupled Enzymatic Reactions. Angew. Chem. Int. Ed. 2022, 61, e202201765. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Ahmed, S.; Ali, M.A. Chitooligosaccharides and their structural-functional effect on hydrogels: A review. Carbohyd. Polym. 2021, 261, 117882. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Kang, H.F.; Bielec, M.; Wu, X.P.; Cheng, Q.; Wei, W.Y.; Dai, H.L. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohyd. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.N.; Xu, X.L.; Wang, C.Z.; Ma, Y.J.; Hui, D.; Zhou, Z.W. Remarkably improvement in antibacterial activity by synergistic effect in n-Cu@T-ZnO nanocomposites. Compos. Part B Eng. 2017, 110, 32–38. [Google Scholar] [CrossRef]
- Mallick, S.P.; Panda, S.P.; Gayatri, A.; Kunaal, Y.; Naresh, C.; Suman, D.K.; Samineni, J.; Siddiqui, N.; Singh, B.N. Chitosan Oligosaccharide Based Hydrogel: An Insight into the Mechanical, Drug Delivery, and Antimicrobial Studies. Biointerface Res. Appl. Chem. 2021, 11, 10293–10300. [Google Scholar]
- Pal, V.K.; Roy, S. Cooperative Metal Ion Coordination to the Short Self-Assembling Peptide Promotes Hydrogelation and Cellular Proliferation. Macromol. Biosci. 2022, 22, 2100462. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, H.Q.; Zhang, B.Y.; Wang, Y.J.; Ren, X.Y.; Guan, S.; Gao, G.H. Highly stretchable and tough pH-sensitive hydrogels with reversible swelling and recoverable deformation. RSC Adv. 2016, 6, 4850–4857. [Google Scholar] [CrossRef]
- Shen, J.L.; Chen, A.; Cai, Z.W.; Chen, Z.J.; Cao, R.C.; Liu, Z.C.; Li, Y.L.; Hao, J. Exhausted local lactate accumulation via injectable nanozyme-functionalized hydrogel microsphere for inflammation relief and tissue regeneration. Bioact. Mater. 2022, 12, 153–168. [Google Scholar] [CrossRef]
- Daoud, L.; Bennour, S. Synthesis and Characterization of Carboxymethyl Cellulose-Graft-Poly(Acrylamide-co-Crotonic Acid) Hydrogel: Matrix for Ammonium Nitrate Release, as Agrochemical. Russ. J. Appl. Chem. 2021, 94, 1499–1512. [Google Scholar] [CrossRef]
- Ma, L.L.; Zhou, Y.L.; Zhang, Z.W.B.; Liu, Y.Q.; Zhai, D.; Zhuang, H.; Li, Q.; Yuye, J.D.; Wu, C.T.; Chang, J. Multifunctional bioactive Nd-Ca-Si glasses for fluorescence thermometry, photothermal therapy, and burn tissue repair. Sci. Adv. 2020, 6, eabb1311. [Google Scholar] [CrossRef]
- Han, Z.L.; Wang, P.; Lu, Y.C.; Jia, Z.; Qu, S.X.; Yang, W. A versatile hydrogel network-repairing strategy achieved by the covalent-like hydrogen bond interaction. Sci. Adv. 2022, 8, eabl5066. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.H.; Liu, L.; Zhang, Z.M.; Song, J.Z.; Li, S.L.; Chen, K.X.; Gao, G.H.; Wang, Y. Tough and self-healing hydrogels based on transient crosslinking by nanoparticles. Soft Matter 2022, 18, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Banza, M.; Rutto, H. Continuous fixed-bed column study and adsorption modeling removal of Ni2+, Cu2+, Zn2+ and Cd2+ ions from synthetic acid mine drainage by nanocomposite cellulose hydrogel. J. Environ. Sci. Health Part A 2022, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kaur, H. Sprayed in-situ synthesis of polyvinyl alcohol/chitosan loaded silver nanocomposite hydrogel for improved antibacterial effects. Int. J. Biol. Macromol. 2020, 145, 950–964. [Google Scholar] [CrossRef]
- Saarai, A.; Sedlacek, T.; Kasparkova, V.; Kitano, T.; Saha, P. On the characterization of sodium alginate/gelatine-based hydrogels for wound dressing. J. Appl. Polym. Sci. 2012, 126, E79–E88. [Google Scholar] [CrossRef]
- El-Kalyoubi, S.A.; Taher, E.S.; Ibrahim, T.S.; El-Behairy, M.F.; Al-Mahmoudy, A.M.M. Uracil as a Zn-Binding Bioisostere of the Allergic Benzenesulfonamide in the Design of Quinoline–Uracil Hybrids as Anticancer Carbonic Anhydrase Inhibitors. Pharmaceuticals 2022, 15, 494. [Google Scholar] [CrossRef]
- Xiaoyu, G.; Bingyuan, Z.; Dongping, L.; Mengyan, H.; Qinxin, H.; Jinming, C. Remediation and resource utilization of chromium(III)-containing tannery effluent based on chitosan-sodium alginate hydrogel. J. Carbohydr. Polym. 2022, 284, 119179. [Google Scholar]
- Mojtaba, M.; Leila, T.; Reza, A.A.; Ali, M. A simple, robust, and efficient structural model to predict thermal stability of zinc metal-organic frameworks (Zn-MOFs): The QSPR approach. J. Microporous Mesoporous Mater. 2022, prepublish. [Google Scholar]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Li, D.R.; Fei, X.; Wang, K.; Xu, L.Q.; Wang, Y.; Tian, J.; Li, Y. A novel self-healing triple physical cross-linked hydrogel for antibacterial dressing. J. Mater. Chem. B 2021, 9, 6844–6855. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Dou, X.Y.; Zhang, L.Y.; Wang, H.F.; Zhang, T.; Bai, R.S.; Sun, Q.N.; Wang, X.; Yu, T.T.; Wu, D.C.; et al. Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact. Mater. 2022, 11, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Bu, Q.; Tao, N.; Chen, M.; Liu, H.; Zhou, J.; Liu, J.; Deng, B.; Kong, N.; Zhang, X.; et al. A facile and general method for synthesis of antibiotic-free protein-based hydrogel: Wound dressing for the eradication of drug-resistant bacteria and biofilms. Bioact. Mater. 2022, 18, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Wang, L.; Lin, Q. Advances of Chitosan/cyclodextrin in Biomedical Material Field. Chem. World 2011, 52, 53–56. [Google Scholar]
- De Masi, A.; Scognamiglio, P.L.; Battista, E.; Netti, P.A.; Causa, F. PEG-based cleavable hydrogel microparticles with controlled porosity for permiselective trafficking of biomolecular complexes in biosensing applications. J. Mater. Chem. B 2022, 10, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Tomic, S.L.J.; Vukovic, J.S. Antimicrobial Activity of Silver, Copper, and Zinc Ions/Poly(Acrylate/Itaconic Acid) Hydrogel Matrices. Inorganics 2022, 10, 38. [Google Scholar] [CrossRef]
- Ma, P.Y.; Wang, Z.; Jiang, Y.B.; Huang, Z.W.; Xia, L.; Jiang, J.L.; Yuan, F.L.; Xia, H.; Zhang, Y. Clay-based nanocomposite hydrogels with microstructures and sustained ozone release for antibacterial activity. Colloid Surf. A 2022, 641, 128497. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Gao, D.; Liu, Y.; Ni, J.; Zhang, Z.; Huang, Y.; Xu, G.; Yang, Z.; Zhang, X.; et al. An NIR photothermal-responsive hybrid hydrogel for enhanced wound healing. Bioact. Mater. 2022, 16, 162–172. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 6, 2100475. [Google Scholar] [CrossRef]
- Xiuhong, H.; Licheng, L.; Xing, Y.; Zhentao, L.; Yi, W.; Lihua, L.; Yanpeng, J.; Yi, Z.; Changren, Z. Chitooligosaccharide-europium (III) functional micron complex with visualized inflammation monitoring, immunomodulation and pro-vascularization activities for effective wound healing of pressure ulcers injury. J. Appl. Mater. Today 2022, 26, 101310. [Google Scholar]
- Liu, Y.; Wang, Q.; Liu, X.; Nakielski, P.; Pierini, F.; Li, X.; Yu, J.; Ding, B. Highly Adhesive, Stretchable and Breathable Gelatin Methacryloyl-based Nanofibrous Hydrogels for Wound Dressings. ACS Appl. Bio Mater. 2022, 5, 1047–1056. [Google Scholar] [CrossRef]
- Gasparini, S.; Fonfara, S.; Kitz, S.; Hetzel, U.; Kipar, A. Canine Dilated Cardiomyopathy: Diffuse Remodeling, Focal Lesions, and the Involvement of Macrophages and New Vessel Formation. Vet. Pathol. 2020, 57, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Valmikinathan, C.M.; Byun, J.; Kim, S.; Lee, G.; Mokarram, N.; Pai, S.B.; Um, E.; Bellamkonda, R.V.; Yoon, Y.S. Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. Biomaterials 2015, 63, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Zhou, Y.J.; Ye, M.Q.; An, Y.; Wang, K.N.; Wu, Q.J.; Song, L.W.; Zhang, J.W.; He, H.C.; Zhang, Q.W.; et al. Freeze-Thawing Chitosan/Ions Hydrogel Coated Gauzes Releasing Multiple Metal Ions on Demand for Improved Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, 2001591. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Holdt, S.L.; Meyer, A.S.; Jacobsen, C. Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus. Mar. Drugs 2022, 20, 263. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, A.; Bianchi, A.; Kahn, C.J.F.; Velot, E.; Arab-Tehrany, E.; Cakir-Kiefer, C.; Linder, M. Encapsulation of Salmon Peptides in Marine Liposomes: Physico-Chemical Properties, Antiradical Activities and Biocompatibility Assays. Mar. Drugs 2022, 20, 249. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Guo, T.; Shi, C.; Zhang, K.; Cui, S.; Zhao, D.; Yang, F.; Chen, J. Preparation of Ion2+-COS/SA Multifunctional Gel Films for Skin Wound Healing by an In Situ Spray Method. Mar. Drugs 2022, 20, 401. https://doi.org/10.3390/md20060401
Chen L, Guo T, Shi C, Zhang K, Cui S, Zhao D, Yang F, Chen J. Preparation of Ion2+-COS/SA Multifunctional Gel Films for Skin Wound Healing by an In Situ Spray Method. Marine Drugs. 2022; 20(6):401. https://doi.org/10.3390/md20060401
Chicago/Turabian StyleChen, Liqi, Tingting Guo, Chao Shi, Kun Zhang, Shenghao Cui, Di Zhao, Faming Yang, and Jingdi Chen. 2022. "Preparation of Ion2+-COS/SA Multifunctional Gel Films for Skin Wound Healing by an In Situ Spray Method" Marine Drugs 20, no. 6: 401. https://doi.org/10.3390/md20060401