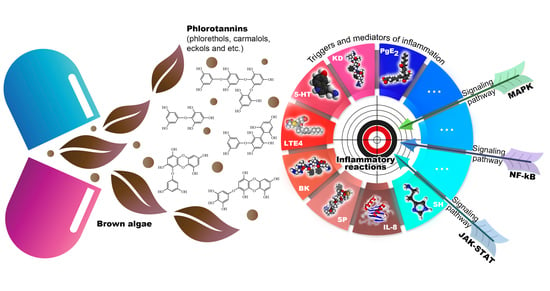
Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins
Abstract
:1. Introduction
2. General Characteristics of Seaweed Polyphenols
2.1. Classification of Phlorotannins
2.2. The Content of PTs in Brown Seaweed
2.3. Extraction and Structure PTs
2.4. Toxicity of Phlorotannins
2.5. Bioavailability of Phlorotannins
3. Anti-Inflammatory Effect of Brown Algae Phlorotannins
3.1. Models for Testing Anti-Inflammatory Compounds
3.2. Molecular Targets of the Anti-Inflammatory Activity of Phlorotannins
3.2.1. Reactive Oxygen Species
3.2.2. NO and NO Synthase
3.2.3. Signaling Pathway Nrf2-Ho-1
3.2.4. TLR-Signaling Pathway
3.2.5. NF-kB-Signaling Pathway
3.2.6. MAPK Signaling Pathway
3.2.7. Arachidonic Acid Signaling Pathway
3.2.8. JAK-STAT Signaling Pathway
3.2.9. Matrix Metalloproteinases (MMP)
3.2.10. Other Targets of Seaweed Phlorotannins
4. Comparison of the Effectiveness of PTs and Known Anti-Inflammatory Drugs
5. Oral Administration of Phlorotannins
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chereshnev, V.A.; Gusev, E.Y. Immunological and Pathophysiological Mechanisms of Systemic Inflammation. Med. Immunol. 2014, 14, 9–20. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Karateev, A.E.; Davydov, O.S. Pain and Inflammation. Part 1. Pathogenetic Aspects. Rheumatol. Sci. Pract. 2017, 54, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Platanitis, E.; Decker, T. Regulatory Networks Involving STATs, IRFs, and NFκB in Inflammation. Front. Immunol. 2018, 9, 2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide Attenuates Inflammatory Response in Membranous Glomerulo-Nephritis Rat via Downregulation of NF-κB Signaling Pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Bogolitsin, K.G.; Druzhinina, A.S.; Ovchinnikov, D.V.; Kaplitsin, P.A.; Shulgin, E.V.; Parshina, A.E. Brown algae polyphenols. Chem. Plant Raw Mater. 2018, 3, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Chen, J.; Wang, P.; Fan, H.; Hou, S.; Gong, Y. Major signaling pathways and key mediators of macrophages in acute kidney injury (Review). Mol. Med. Rep. 2021, 23, 455. [Google Scholar] [CrossRef]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 23061. [Google Scholar] [CrossRef] [Green Version]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Katsuzaki, H.; Imai, K.; Amano, H. The Anti-Allergic and Anti-Inflammatory Effects of Phlorotannins from the Edible Brown Algae, Ecklonia sp. and Eisenia sp. Nat. Prod. Commun. 2021, 16, 12. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; Fitzgerald, R.J.; Smyth, T.J. Profiling of the Molecular Weight and Structural Isomer Abundance of Macroalgae-Derived Phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Barroso, S.; Ferreira, A.; Adão, P.; Mendes, S.; Gil, M. Seasonal Evaluation of Phlorotannin-Enriched Extracts from Brown Macroalgae Fucus Spiralis. Molecules 2021, 26, 4287. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 61327. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Corona, G.; Coman, M.M.; Guo, Y.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Effect of simulated gastrointestinal digestion and fermentation on polyphenolic content and bioactivity of brown seaweed phlorotannin-rich extracts. Mol. Nutr. Food Res. 2017, 61, 1700223. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Bonham, M.P.; Ryan, L. Do marine algal polyphenols have antidiabetic, antihyperlipidemic or anti-inflammatory effects in humans? A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2039–2054. [Google Scholar] [CrossRef]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.; Pereira, L.; Gonçalves, A. Environmental Impact on Seaweed Phenolic Production and Activity: An Important Step for Compound Exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef]
- Lee, S.-H.; Eom, S.-H.; Yoon, N.-Y.; Kim, M.-M.; Li, Y.-X.; Ha, S.K.; Kim, S.-K. Fucofuroeckol-A from Eisenia bicyclis Inhibits Inflammation in Lipopolysaccharide-Induced Mouse Macrophages via Down-regulation of the MAPK/NF-κB Signaling Pathway. J. Chem. 2016, 2016, 6509212. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Miyata, M. Anti-inflammatory effects of 6,6′-bieckol and 6,8′-bieckol from Esenia arborea on mouse ear swelling. Food Sci. Technol. Res. 2017, 23, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Erpel, F.; Mateos, R.; Perez-Jimenez, J.; Perez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef] [PubMed]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10, S2608–S2614. [Google Scholar] [CrossRef] [Green Version]
- Jégou, C.; Kervarec, N.; Cérantola, S.; Bihannic, I.; Stiger-Pouvreau, V. NMR use to quantify phlorotannins: The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France). Talanta 2015, 135, 1–6. [Google Scholar] [CrossRef]
- Kim, J.; Um, M.; Yang, H.; Kim, I.; Lee, C.; Kim, Y.; Yoon, M.; Kim, Y.; Kim, J.; Cho, S. Method development and validation for dieckol in the standardization of phlorotannin preparations. Fish. Aquat. Sci. 2016, 19, 3. [Google Scholar] [CrossRef] [Green Version]
- Phasanasophon, K.; Kim, S.M. Anti-Inflammatory Activity of the Phlorotannin Trifuhalol A Using LPSStimulated RAW264.7 Cells Through NF-κB and MAPK Main Signaling Pathways. Nat. Prod. Commun. 2019, 14, 1934578X19849798. [Google Scholar] [CrossRef] [Green Version]
- Sardari, R.R.R.; Prothman, J.; Gregerson, G.; Turner, C.; Karlsson, E.N. Identification of florotannins in the brown algae, Saccharina latissima and Ascophyllum nodosum by ultra-high-perfomance liquid chromatography coupled to high-resolution tandem mass spectrometry. Molecules 2021, 26, 43. [Google Scholar] [CrossRef]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Gaspar, H.; Pinteus, S.; Mouga, T.; Goettert, M.; Petrovski, Ž.; Branco, L.; et al. Unravelling the Dermatological Potential of the Brown Seaweed Carpomitra costata. Mar. Drugs 2021, 19, 135. [Google Scholar] [CrossRef]
- Saravana, P.S.; Getachew, A.T.; Cho, Y.-J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Scheldrake, G.N.; Walsh, P.J. A critical reviews of analytical methods used for the chemical characterization and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Vanuopadath, M.; Balasubramanian, A.; Iyer, A.; Ganesh, S.; Anil, A.N.; Vikraman, V.; Pillai, P.; Bose, C.; Nair, B.; et al. Phlorotannins from Padina tetrastromatica: Structural characterisation and functional studies. J. Appl. Phycol. 2019, 31, 3131–3141. [Google Scholar] [CrossRef]
- Gager, L.; Connan, S.; Molla, M.; Couteau, C.; Arbona, J.-F.; Coiffard, L.; Cérantola, S.; Stiger-Pouvreau, V. Active phlorotannins from seven brown seaweeds commercially harvested in Brittany (France) detected by 1H NMR and in vitro assays: Temporal variation and potential valorization in cosmetic applications. J. Appl. Phycol. 2020, 32, 2375–2386. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Kim, A.-R.; Lee, M.-S.; Shin, T.-S.; Hua, H.; Jang, B.-C.; Choi, J.-S.; Byun, D.-S.; Utsuki, T.; Ingram, D.; Kim, H.-R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef]
- Yang, Y.-I.; Shin, H.-C.; Kim, S.H.; Park, W.-Y.; Lee, K.-T.; Choi, J.-H. 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE2 production and inflammatory cytokine expression in macrophages: The inhibition of NFκB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef]
- Yu, D.-K.; Lee, B.; Kwon, M. Phlorofucofuroeckol B suppresses inflammatory responses by down-regulating nuclear factor κB activation via Akt, ERK, and JNK in LPS-stimulated microglial cells. Int Immunopharmacol. 2015, 28, 1068–1075. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Eom, S.-H.; Kim, Y.-M. Protective effect of phlorotannins from Eisenia bicyclis against lipopolysaccharide-stimulated inflammation in HepG2 cells. Environ. Toxicol. Pharmacol. 2013, 35, 395–401. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2020, 39, 100832. [Google Scholar] [CrossRef]
- Negara, B.; Sohn, J.; Kim, J.-S.; Choi, J.-S. Effects of Phlorotannins on Organisms: Focus on the Safety, Toxicity, and Availability of Phlorotannins. Foods 2021, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Paradis, M.-E.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a polyphenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-week Oral Supplementation of Ecklonia cava Polyphenols on Anthropometric and Blood Lipid Parameters in Overweight Korean Individuals: A Double-blind Randomized Clinical Trial. Phytother. Res. 2011, 26, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Kim, J.Y.; Han, J.K.; Kim, J.; Yang, H.; Yoon, M.; Kim, J.; Kang, S.W.; Cho, S. Phlorotannin supplement decreases wake after sleep onset in adults with self-reported sleep disturbance: A randomized, controlled, dooble-blind clinical and polysomnographic study. Phytother. Res. 2018, 32, 698–704. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA); Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; et al. Safety of Ecklonia cava phlorotannins as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e05003. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Espiín, J.C.; Tomaás-Barberaán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Kim, A.-R.; Shin, T.-S.; Lee, M.-S.; Park, J.-Y.; Park, K.-E.; Yoon, N.-Y.; Kim, J.-S.; Choi, J.-S.; Jang, B.-C.; Byun, D.-S.; et al. Isolation and Identification of Phlorotannins from Ecklonia stolonifera with Antioxidant and Anti-inflammatory Properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Ahn, G.; Lee, W.-W.; Kang, M.-C.; Kim, E.-A.; Jeon, Y.-J. Anti-inflammatory activity of phlorotannin-rich fermented Ecklonia cava processing by-product extract in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Appl. Phycol. 2012, 25, 1207–1213. [Google Scholar] [CrossRef]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria pinnatifida Sporophyll: Extraction, Antioxidant, and Anti-Inflammatory Activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, K.; Fu, J. HDAC6 Mediates Macrophage iNOS Expression and Excessive Nitric Oxide Production in the Blood During Endotoxemia. Front. Immunol. 2020, 11, 1893. [Google Scholar] [CrossRef]
- Tucureanu, M.M.; Rebleanu, D.; Constantinescu, C.A.; Deleanu, M.; Voicu, G.; Butoi, E.; Calin, M.; Manduteanu, I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int. J. Nanomed. 2017, 13, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, Y.; Tanaka, R.; Katsuzaki, H.; Imai, K.; Matsushita, T. The anti-inflammatory effect of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J. Funct. Foods 2013, 5, 2019–2023. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, M.; Weinberg, S.; Chandel, N.S. Mitochondrial control of immunity: Beyond ATP. Nat. Rev. Immunol. 2017, 17, 608–620. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2013, 395, 203–230. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative Eustress and Oxidative Distress. In Oxidative Stress; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Silva, S.D.; Jara, Z.P.; Peres, R.; Lima, L.S.; Scavone, C.; Montezano, A.C.; Touyz, R.M.; Casarini, D.; Michelini, L.C. Temporal changes in cardiac oxidative stress, inflammation and remodeling induced by exercise in hypertension: Role for local angiotensin II reduction. PLoS ONE 2017, 12, e0189535. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular Mechanisms behind Free Radical Scavengers Function against Oxidative Stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Liu, J.-F.; Chen, P.-C.; Chang, T.-M.; Hou, C.-H. Monocyte Chemoattractant Protein-1 promotes cancer cell migration via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. J. Exp. Clin. Cancer Res. 2020, 39, 254. [Google Scholar] [CrossRef] [PubMed]
- Cherubim, D.J.D.L.; Martins, C.V.B.; Fariña, L.O.; Lucca, R.A.D.S.D. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2019, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Ahn, G. Marine algal flavonoids and phlorotannins; an intriguing frontier of biofunctional secondary metabolites. Crit. Rev. Biotechnol. 2022, 42, 23–45. [Google Scholar] [CrossRef]
- Han, J.; Jiang, Y.; Li, Z.; Kravchenko, V.V.; Ulevitch, R.J. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 1997, 386, 296–299. [Google Scholar] [CrossRef]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts. Antioxidants 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Rubika, J.J.; Pandiarajan, J. In vitro antioxidant study of polyphenol from red seaweeds dichotomously branched gracilaria Gracilaria edulis and robust sea moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411. [Google Scholar] [CrossRef]
- Blamo, P.A.; Pham, H.N.T.; Nguyen, T.H. Maximising phenolic compounds and antioxidant capacity from Laurencia intermedia using ultrasound-assisted extraction. AIMS Agric. Food 2021, 6, 32–48. [Google Scholar] [CrossRef]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown Algae Phlorotannins: A Marine Alternative to Break the Oxidative Stress, Inflammation and Cancer Network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef]
- Kirke, D.; Smyth, T.; Rai, D.; Kenny, O.; Stengel, D. The chemical and antioxidant stability of isolated low molecular weight phlorotannins. Food Chem. 2016, 221, 1104–1112. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds against Oxidative Stress-Associated Pathology. Oxid. Med. Cell. Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2007, 20, 705–711. [Google Scholar] [CrossRef]
- Han, Y.R.; Ali, M.Y.; Woo, M.-H.; Jung, H.A.; Choi, J.S. Anti-diabetic and anti-inflammatory potential of the edible brown alga Hizikia fusiformis. J. Food Biochem. 2015, 39, 417–428. [Google Scholar] [CrossRef]
- Nakai, M.; Kageyama, N.; Nakahara, K.; Miki, W. Phlorotannins as Radical Scavengers from the Extract of Sargassum ringgoldianum. Mar. Biotechnol. 2006, 8, 409–414. [Google Scholar] [CrossRef]
- Rajput, V.; Singh, R.K.; Verma, K.; Sharma, L.; Quiroz-Figueroa, F.; Meena, M.; Gour, V.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Demchenko, S.A.; Koklin, I.S.; Koklina, N.Y. Role of Arginase 2 as a potential pharmacological target for the creation of new drugs to correct cardiovascular diseases. Res. Results Pharmacol. 2020, 6, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Paolo, S. Nitric Oxide in Human Health and Disease. Intern J. Cell Biol. 2012, 29, 571067. [Google Scholar] [CrossRef] [Green Version]
- Forstermann, U.; Sessa, W. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.; Cruz, M.T.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of Inflammatory Response by Blocking NF-κB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 8697. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.; Brunton, N.P.; Smyth, T.J. In Vitro Protocols for Measuring the Antioxidant Capacity of Algal Extracts. Methods Mol Biol. 2015, 1308, 375–402. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Wetzel, M.D.; Stanley, K.; Wang, W.W.; Maity, S.; Madesh, M.; Reeves, W.B.; Awad, A.S. Selective inhibition of arginase-2 in endothelial cells but not proximal tubules reduces renal fibrosis. JCI Insight 2020, 5, e142187. [Google Scholar] [CrossRef]
- Minozzo, B.; Fernandes, D.; Beltrame, F. Phenolic Compounds as Arginase Inhibitors: New Insights Regarding Endothelial Dysfunction Treatment. Planta Med. 2018, 84, 277–295. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, M.A.; Didelija, I.C.; Marini, J.C. Arginase II Plays a Central Role in the Sexual Dimorphism of Arginine Metabolism in C57BL/6 Mice. J. Nutr. 2020, 150, 3133–3140. [Google Scholar] [CrossRef]
- Dhillon, S.S.; Mahadevan, K.; Bandi, V.; Zheng, Z.; Smith, C.W.; Rumbaut, R.E. Neutrophils, Nitric Oxide, and Microvascular Permeability in Severe Sepsis. Chest 2005, 128, 1706–1712. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2020, 27, 314–331. [Google Scholar] [CrossRef]
- Kim, T.H.; Ku, S.-K.; Bae, J.-S. Antithrombotic and profibrinolytic activities of eckol and dieckol. J. Cell. Biochem. 2012, 113, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264. 7 macrophages. Food Chem Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Cha, H.-J.; Choi, E.O.; Kim, C.H.; Kim, G.-Y.; Yoo, Y.H.; Hwang, H.-J.; Park, H.T.; Yoon, H.M.; Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int. J. Med. Sci. 2019, 16, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, Z.; Koh, Y.-S. Mitogen-activated Protein Kinases in Inflammation. J. Bacteriol. Virol. 2012, 42, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Tovar, E.; Muriel, P. Free radicals, antioxidants, nuclear factor-E2-related factor-2 and liver damage. J. Appl. Toxicol. 2019, 40, 151–168. [Google Scholar] [CrossRef]
- Mendonca, P.; Soliman, K.F.A. Flavonoids Activation of the Transcription Factor Nrf2 as a Hypothesis Approach for the Prevention and Modulation of SARS-CoV-2 Infection Severity. Antioxidants 2020, 9, 659. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Stress-Activated Capncollar Transcription Factors in Aging and Human Disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Jun, Y.-J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.-H.; Kim, H.-R. Eckol Enhances Heme Oxygenase-1 Expression through Activation of Nrf2/JNK Pathway in HepG2 Cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.-J.; Hyun, J.W. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int. J. Biochem. Cell Biol. 2010, 42, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.; Palosaari, S.; Abdelwahab, S.A.; Rifaai, R.A.; El-Tahawy, N.F.; Saber, E.A.; Nousiainen, T.; Valkealahti, M.; Huhtakangas, J.; Karttunen, T.J.; et al. Differential synovial tissue expression of TLRs in seropositive and seronegative rheumatoid arthritis: A preliminary report. Autoimmunity 2020, 54, 23–34. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.-R.; Byeon, Y.; Kim, D.; Park, S.-G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.M.; Majidi, J.; Baradaran, B.; Yousefi, M. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases. Adv. Pharm. Bull. 2015, 5, 605–614. [Google Scholar] [CrossRef]
- Takagi, M.; Takakubo, Y.; Pajarinen, J.; Naganuma, Y.; Oki, H.; Maruyama, M.; Goodman, S.B. Danger of frustrated sensors: Role of Toll-like receptors and NOD-like receptors in aseptic and septic inflammations around total hip replacements. J. Orthop. Transl. 2017, 10, 68–85. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, J.C. The Toll receptor family and microbial recognition. Trends Microbiol. 2000, 8, 452–456. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Itav, S.; Elinav, E. Integration of Innate Immune Signaling. Trends Immunol. 2016, 37, 84–101. [Google Scholar] [CrossRef]
- Nie, F.; Ding, F.; Chen, B.; Huang, S.; Liu, Q.; Xu, C. Dendritic cells aggregate inflammation in experimental osteoarthritis through a toll-like receptor (TLR)-dependent machinery response to challenges. Life Sci. 2019, 238, 116920. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Son, M.; Choi, J.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Phlorotannins from Ecklonia cava Attenuates Palmitate-Induced Endoplasmic Reticulum Stress and Leptin Resistance in Hypothalamic Neurons. Mar. Drugs 2019, 17, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-κB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. WIREs Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [Green Version]
- De Jesus, T.J.; Ramakrishnan, P. NF-kB c-Rel dictates the inflammatory threshold by acting as a transcriptional repressor. Science 2020, 23, 100876. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, G.; Wang, V.Y.; Huang, D.-B.; Fusco, A. NF-kB-regulation: Lessons from structures. Immunol. Rev. 2012, 246, 36–58. [Google Scholar] [CrossRef] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-ΚB P65 and Strategies for Therapeutic Manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.E.; Jung, Y.C.; Jung, I.; Lee, H.; Youn, H.; Lee, J.S. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C Agardh on lipopolysaccharide-stimulated macrophage activation via NF-kB pathway regulation. J. Mol. Cell. Immunol. 2015, 44, 137–146. [Google Scholar]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.-R.; Lee, B.; Joung, E.-J.; Gwon, W.-G.; Utsuki, T.; Kim, N.-G.; Kim, H.-R. 6,6′-Bieckol suppresses inflammatory responses by down-regulating nuclear factor-κB activation via Akt, JNK, and p38 MAPK in LPS-stimulated microglial cells. Immunopharmacol. Immunotoxicol. 2016, 38, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. [Google Scholar] [CrossRef]
- Yang, B.; Li, Y.; Yang, Z.; Xue, L.; Zhang, M.; Chen, G.; Chinnathambi, A.; Alahmadi, T.A.; Liu, X. Antiinflammatory and ant-cell-proliferative effects of dieckol in the prevention and treatment of colon cancer induced by 1,2-dimethyl hydrazine in experimental animals. Pharmacogn. Mag. 2020, 16, 851–858. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochem. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Helou, D.G.; Martin, S.F.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Römer, S. Nrf2 involvement in chemical-induced skin innate immunity. Front. Immunol. 2019, 10, 1004. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-I.; Woo, J.-H.; Seo, Y.-J.; Lee, K.-T.; Lim, Y.; Choi, J.-H. Protective Effect of Brown Alga Phlorotannins against Hyper-inflammatory Responses in Lipopolysaccharide-Induced Sepsis Models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef]
- Ahmadabad, R.A.; Ghadiri, M.K.; Gorji, A. The role of Toll-like receptor signaling pathways in cerebrovascular disorders: The impact of spreading depolarization. J. Neuroinflamm. 2020, 17, 108. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-kB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, S.-S.; Choi, S.-I.; Kim, G.-H.; Imm, J.-Y. Ecklonia cava Extract Containing Dieckol Suppresses RANKL-Induced Osteoclastogenesis via MAP Kinase/NF-kB Pathway Inhibition and Heme Oxygenase-1 Induction. J. Microbiol. Biotechnol. 2019, 29, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.-K.; Ahn, Y.-W.; Lee, S.-H.; Choi, Y.H.; Kim, S.-K.; Yea, S.S.; Choi, I.; Park, S.-G.; Seo, S.-K.; Lee, S.-W.; et al. Ecklonia cava ethanolic extracts inhibit lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV2 microglia via the MAP kinase and NF-κB pathways. Food Chem. Toxicol. 2009, 47, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Cha, S.-H.; Ko, J.-Y.; Kang, M.-C.; Kim, D.; Heo, S.-J.; Kim, J.-S.; Heu, M.S.; Kim, Y.-T.; Jung, W.-K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between systemic inflammation and neuroinflammation underlies neuro-protective mechanism of several phytochemicals in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanism of nuclear translocation. Biochim. Biophys. Acta 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.J.; Kim, M.-Y.; Cho, J.Y. MAPK/AP-1-Targeted Anti-Inflammatory Activities of Xanthium strumarium. Am. J. Chin. Med. 2016, 44, 1111–1125. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK Signal Transduction Pathways Activated by Stress and Inflammation: A 10-Year Update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [Green Version]
- Horton, A.A.; Wang, B.; Camp, L.; Price, M.S.; Arshi, A.; Nagy, M.; Nadler, S.A.; Faeder, J.R.; Luckhart, S. The mitogen-activated protein kinome from Anopheles gambiae: Identification, phylogeny and functional characterization of the ERK, JNK and p38 MAP kinases. BMC Genom. 2011, 12, 574. [Google Scholar] [CrossRef] [Green Version]
- Ruan, W.; Ji, X.; Qin, Y.; Zhang, X.; Wan, X.; Zhu, C.; Lv, C.; Hu, C.; Zhou, J.; Lu, L.; et al. Harmine Alleviated Sepsis-Induced Cardiac Dysfunction by Modulating Macrophage Polarization via the STAT/MAPK/NF-κB Pathway. Front. Cell Dev. Biol. 2022, 9, 792257. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Garces de los Fayos Alonso, I.; Liang, H.-C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazon, H.; Barbeau, B.; Mesnard, J.-M.; Peloponese, J.-M., Jr. Hijacking of the AP-1 Signaling Pathway during Development of ATL. Front. Microbiol. 2018, 8, 2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [Green Version]
- El Zaoui, I.; Bucher, M.; Rimoldi, D.; Nicolas, M.; Kaya, G.; Gobert, R.P.; Bedoni, N.; Schalenbourg, A.; Sakina, E.; Zografos, L.; et al. Conjunctival Melanoma Targeted Therapy: MAPK and PI3K/mTOR Pathways Inhibition. Investig. Opthalmol. Vis. Sci. 2019, 60, 2764–2772. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal. Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Guaita, R.; Pichiule, M.; Maté, T.; Linares, C.; Díaz, J. Short-term impact of particulate matter (PM2.5) on respiratory mortality in Madrid. Int. J. Environ. Health Res. 2011, 21, 260–274. [Google Scholar] [CrossRef]
- Halonen, J.I.; Lanki, T.; Yli-Tuomi, T.; Tiittanen, P.; Kulmala, M.; Pekkanen, J. Particulate Air Pollution and Acute Cardiorespiratory Hospital Admissions and Mortality Among the Elderly. Epidemiology 2009, 20, 143–153. [Google Scholar] [CrossRef]
- Perez, L.; Tobías, A.; Querol, X.; Pey, J.; Alastuey, A.; Diaz, J.; Sunyer, J. Saharan dust, particulate matter and cause-specific mortality: A case–crossover study in Barcelona (Spain). Environ. Int. 2012, 48, 150–155. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Fernando, P.D.S.M.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol Inhibits Particulate Matter 2.5-Induced Skin Keratinocyte Damage via MAPK Signaling Pathway. Mar. Drugs 2019, 17, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Lee, Y.; Kim, S.Y.; Kim, H.S.; Joo, H.G.; Park, J.W.; et al. Eckol isolated from Ecklonia cava attenuates oxidative stress induced cell damage in lung fibroblast cells. FEBS Lett. 2005, 579, 6295–6304. [Google Scholar] [CrossRef] [Green Version]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W. The inhibitory effects of eckol and dieckol from E. stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharmacol. Bull. 2006, 28, 1735–1739. [Google Scholar] [CrossRef]
- Shibata, T.; Nagayama, K.; Tanaka, R.; Yamaguchi, K.; Nakamura, T. Inhibitory effects of brown algal phlorotannins on secretory phospholipase A2s, lipoxygenases and cyclooxygenases. J. Appl. Phycol. 2003, 15, 61–66. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Kurihara, H.; Konno, R.; Takahashi, K. Fucophlorethol C, a phlorotannin as a lipoxygenase inhibitor. Biosci. Biotechnol. Biochem. 2015, 79, 1954–1956. [Google Scholar] [CrossRef] [Green Version]
- Rao, T.; Currie, J.L.; Shaffer, A.F.; Isakson, P.C. Comparative evaluation of arachidonic acid (AA)- and tetradecanoylphorbol acetate (TPA)-induced dermal inflammation. Inflammation 1993, 17, 723–741. [Google Scholar] [CrossRef]
- Rumyantsev, A.; Demina, O. Molecular pathways of JAK in the pathogenesis of inflammation in acne. Immunologiya 2021, 42, 38–48. [Google Scholar] [CrossRef]
- Hu, X.; Li, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Q.; Wang, L.; Yu, H.; Chen, D.; Zhu, W.; Sun, C. Pharmacological Effects of Polyphenol Phytochemicals on the JAK-STAT Signaling Pathway. Front. Pharmacol. 2021, 12, 716672. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Xu, J. Curcumin targets the TFEB-lysosome pathway for induction of autophagy. Oncotarget 2016, 7, 75659–75671. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.-J.; Koo, D.-H.; Kang, G.-J.; Han, S.-C.; Lee, B.-W.; Koh, Y.-S.; Hyun, J.-W.; Lee, N.-H.; Ko, M.-H.; Kang, H.-K.; et al. Dieckol, a Component of Ecklonia cava, Suppresses the Production of MDC/CCL22 via Down-Regulating STAT1 Pathway in Interferon-γ Stimulated HaCaT Human Keratinocytes. Biomol. Ther. 2015, 23, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem. Interact. 2009, 179, 192–201. [Google Scholar] [CrossRef]
- Thomas, N.V.; Diyya, A.S.M.; Ghafour, D.D.; Kim, S.K. Marine Algal Phlorotannins and Their Biological Importance. In Encyclopedia of Marine Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 5, pp. 1535–1558. [Google Scholar] [CrossRef]
- Fingleton, B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta BBA Bioenerg. 2017, 1864, 2036–2042. [Google Scholar] [CrossRef]
- Tikhaeva, K.; Rogova, L.N.; Tkachenko, L. The role of metalloproteinases in the exchange of endometrial extracellular matrix proteins in normal and pathological conditions. Probl. Reproduktsii 2020, 26, 22–29. [Google Scholar] [CrossRef]
- Shadrina, A.S.; Plieva, Y.Z.; Kushlinskiy, D.N.; Morozov, A.A.; Filipenko, M.L.; Chang, V.L.; Kushlinskii, N.E. Classification, regulation of activity, and genetic polymorphism of matrix metalloproteinases in health and disease. Alm. Clin. Med. 2017, 45, 266–279. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A.M. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.; Jouini, M.; Amor, H.B.H.; Mzoughi, Z.; Dridi, M.; Ben Said, R.; Bouraoui, A. Phytochemical Analysis and Evaluation of the Antioxidant, Anti-Inflammatory, and Antinociceptive Potential of Phlorotannin-Rich Fractions from Three Mediterranean Brown Seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Miyata, M. Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling. Mar. Drugs 2018, 16, 267. [Google Scholar] [CrossRef] [Green Version]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 53630. [Google Scholar] [CrossRef] [Green Version]
- Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflaamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef]
- Yoshino, K.; Yamazaki, K.; Sano, M. Preventive effects of black tea theaflavins against mouse type IV allergy. J. Sci. Food Agric. 2010, 90, 1983–1987. [Google Scholar] [CrossRef]
- Nagano, T.; Wu, W.; Tsumura, K.; Yonemoto-Yano, H.; Kamada, T.; Haruma, K. The inhibitory effect of soybean and soybean isoflavone diets on 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Biosci. Biotechnol. Biochem. 2016, 80, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-J.; Park, J.-H.; Lee, B.H.; Chee, H.Y.; Lee, K.B.; Oh, S.-M. Suppression of NF-κB by Dieckol Extracted from Ecklonia cava Negatively Regulates LPS Induction of Inducible Nitric Oxide Synthase Gene. Appl. Biochem. Biotechnol. 2014, 173, 957–967. [Google Scholar] [CrossRef]
- Okeke, E.S.; Nweze, E.J.; Chibuogwu, C.C.; Anaduaka, E.G.; Chukwudozie, K.; Ezeorba, T.P.C. Aquatic Phlorotannins and Human Health: Bioavailability, Toxicity, and Future Prospects. Nat. Prod. Commun. 2021, 16, 1934578X211056144. [Google Scholar] [CrossRef]
- Tong, T.; Liu, X.; Yu, C. Extraction and Nano-Sized Delivery Systems for Phlorotannins to Improve Its Bioavailability and Bioactivity. Mar. Drugs 2021, 19, 625. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Park, S.; Kang, J.; Kim, J.; Yoo, S.; Kim, D.-O.; Kim, G.-H.; Heo, H. Mixture of Phlorotannin and Fucoidan from Ecklonia cava Prevents the Aβ-Induced Cognitive Decline with Mitochondrial and Cholinergic Activation. Mar. Drugs 2021, 19, 434. [Google Scholar] [CrossRef] [PubMed]




| Main Targets and Strategies of Influence of Phlorotannins | References |
|---|---|
| Inactivation of LPS-induced transcriptional activity of NF-kB (dieckol E. cava). | [121,122] |
| Reduced expression of pro-inflammatory proteins: iNOS, pro-IL-Iβ and COX-2 (PT F. vesiculosus) | [85,123,124,125] |
| Inhibition of phosphorylation and degradation of protein- inhibitor IκBα (PT F. vesiculosus) | [126,127] |
| AKT/IkB-mediated inactivation signaling pathway NF-kB (dieckol E. cava) | [128] |
| Decreased production of pro-inflammatory cytokines TNFa and IL-6 (fucofuroeckol-A Eisenia bicyclis) | [129] |
| Reduction of overproduction of NO and PGP2 (trifuhalol A Agarum cribrosum) | [27] |
| Decreased activation of nuclear factor (NF-kB) and mitogen-activated proteinases (MAPKs) | [129,130] |
| Nrf2 and NF-kB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. | [39,131,132] |
| Decreased expression of mRNA and proteins iNOS, COX-2 (PT Ecklonia sp. and Eisenia sp.) | [12,55] |
| Main Targets and Strategies of Influence of Phlorotannins | References |
|---|---|
| Attenuation of MAPK signaling pathway activation due to the inhibition of p38 phosphorylation (trifuhalol, Agarum cribrosum). | [39] |
| Inhibited gene mRNA levels and protein expression of MMP-1, MMP-3 and MMP-13, iNOS and COX-2, and reduced inflammation via the MAPK signaling pathway (dieckol E. cava). | [137] |
| Inhibition of the MAPK signaling pathway has been confirmed by the use of MAPK inhibitors (UO126, SB203580, and SP600125) (eckol E. cava). | [39] |
| Inhibition of phosphorylation and degradation of protein-inhibitor IκBα (eckol F. vesiculosus). | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kuznetsova, T.A.; Kryzhanovsky, S.P.; Ermakova, S.P.; Galkina, I.V.; Shchelkanov, M.Y. Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins. Mar. Drugs 2022, 20, 243. https://doi.org/10.3390/md20040243
Besednova NN, Andryukov BG, Zaporozhets TS, Kuznetsova TA, Kryzhanovsky SP, Ermakova SP, Galkina IV, Shchelkanov MY. Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins. Marine Drugs. 2022; 20(4):243. https://doi.org/10.3390/md20040243
Chicago/Turabian StyleBesednova, Natalya N., Boris G. Andryukov, Tatyana S. Zaporozhets, Tatyana A. Kuznetsova, Sergey P. Kryzhanovsky, Svetlana P. Ermakova, Irina V. Galkina, and Mikhail Yu. Shchelkanov. 2022. "Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins" Marine Drugs 20, no. 4: 243. https://doi.org/10.3390/md20040243