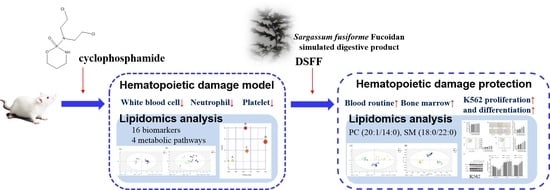
Stimulating the Hematopoietic Effect of Simulated Digestive Product of Fucoidan from Sargassum fusiforme on Cyclophosphamide-Induced Hematopoietic Damage in Mice and Its Protective Mechanisms Based on Serum Lipidomics
Abstract
:1. Introduction
2. Results
2.1. Protective Effect of DSFF on Body Weight and Immune Organ Index in Hematopoietic Damage Mice
2.2. Effect of DSFF on Blood Routine in Cyclophosphamide-Induced Hematopoietic Damage Mice
2.2.1. Restorative Effect of DSFF on White Blood Cell Count
2.2.2. Restorative Effect of DSFF on Neutrophil Count
2.2.3. Restorative Effect of DSFF on Platelet Count
2.3. Protective Effect of DSFF on Bone Marrow DNA Content
2.4. The Hematopoietic Damage Protection Mechanism of DSFF Based on Serum Lipidomics
2.4.1. Screening of Potential Lipid Biomarkers for Hematopoietic Damage in Mouse Serum
2.4.2. Identification of Potential Lipid Biomarkers for DSFF Protective Effects
2.5. Promoting Effect of DSFF on Proliferation and Differentiation of Myeloid K562 Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Hematopoietic Damage Model Establishment and Treatment
4.3. Detection of Relevant Indicators
4.3.1. Examination of Blood Routine
4.3.2. Detection of DNA Content from Bone Marrow
4.4. Serum Samples Preparation for Lipidomics
4.5. UPLC Q-Exactive Orbitrap MS/MS Analysis
4.6. K562 Cell Proliferation Assay
4.7. Differentiation Assay of K562 Cells Using Benzidine Staining and Flow Cytometry
4.8. Western Blotting Detects Differentiation-Related Protein Expression in K562 Cells
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wang, X.L.; He, D.H.; Cheng, Y.X. Protection against chemotherapy- and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.L.; Wang, L.Y.; Wang, J.X.; Wang, C.; Wang, C.L.; Zhao, D.P.; Wang, Z.C.; Zhang, J.J. Protective effects of paeoniflorin and albiflorin on chemotherapy-induced myelosuppression in mice. Chin. J. Nat. Med. 2016, 14, 599–606. [Google Scholar] [CrossRef]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef]
- Myles, N.; Myles, H.; Clark, S.R.; Bird, R.; Siskind, D. Use of granulocyte-colony stimulating factor to prevent recurrent clozapine-induced neutropenia on drug rechallenge: A systematic review of the literature and clinical recommendations. Aust. N. Z. J. Psychiatry 2017, 51, 980–989. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Kudo, H.; Suzuki, S.; Sassa, S.; Mano, Y.; Sakamoto, S. Splenomegaly induced by recombinant human granulocyte-colony stimulating factor in rats. Life Sci. 2001, 69, 1521–1529. [Google Scholar] [CrossRef]
- Giri, N.; Pitel, P.A.; Green, D.; Alter, B.P. Splenic peliosis and rupture in patients with dyskeratosis congenita on androgens and granulocyte colony-stimulating factor. Br. J. Haematol. 2007, 138, 815–817. [Google Scholar] [CrossRef]
- Wu, F.; Huang, H. Surface morphology and protective effect of Hericium erinaceus polysaccharide on cyclophosphamide-induced immunosuppression in mice. Carbohydr. Polym. 2021, 251, 116930. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Niu, Q.; Li, S.; Zhang, X.; Liu, C.; Cai, C.; Li, G.; Yu, G. Fucoidan from sea cucumber Holothuria polii: Structural elucidation and stimulation of hematopoietic activity. Int. J. Biol. Macromol. 2020, 154, 1123–1131. [Google Scholar] [CrossRef]
- Anisimova, N.; Ustyuzhanina, N.; Bilan, M.; Donenko, F.; Usov, A.; Kiselevskiy, M.; Nifantiev, N. Fucoidan and fucosylated chondroitin sulfate stimulate hematopoiesis in cyclophosphamide-induced mice. Mar. Drugs 2017, 15, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.P.; Li, H.H.; Liu, M.; Liu, H.B. Effects of simulated digestion in vitro on the structure and macrophages activation of fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2021, 272, 118484. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.A.; Li, Z.; Rice, S.; Krieg, C.; Fragkogianni, S.; Robinson, M.; Vink, C.S.; Pollard, J.W.; Dzierzak, E. Pro-inflammatory aorta-associated macrophages are involved in embryonic development of hematopoietic stem cells. Immunity 2019, 50, 1439–1452.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, L.; Mumau, M.; Speck, N.A. Macrophages fertilize the soil to promote hematopoietic cell growth. Immunity 2019, 50, 1342–1344. [Google Scholar] [CrossRef]
- Smith, J.N.P.; Dawson, D.M.; Christo, K.F.; Jogasuria, A.P.; Cameron, M.J.; Antczak, M.I.; Ready, J.M.; Gerson, S.L.; Markowitz, S.D.; Desai, A.B. 15-PGDH inhibition activates the splenic niche to promote hematopoietic regeneration. JCI Insight 2021, 6, e143658. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Laiakis, E.C.; Singh, V.K.; Fornace, A.J. Lipidomic signatures of nonhuman primates with radiation-induced hematopoietic syndrome. Sci. Rep. 2017, 7, 9777. [Google Scholar] [CrossRef] [Green Version]
- Yildirim Simsir, I.; Donmez, A.; Kabaroglu, C.; Yavasoglu, I.; Basol, G.; Gungor, A.; Comert Ozkan, M.; Saygili, F.; Bolaman, Z.; Tombuloglu, M. The effect of serum lipid levels on peripheral blood hematopoietic stem cell levels. Transfus. Apher. Sci. 2021, 60, 103074. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies gut microbiota and intestinal metabolites during alleviation of hyperglycemia in type 2 diabetic mice. Food Funct. 2021, 12, 3572–3585. [Google Scholar] [CrossRef]
- Lei, G.; Xi, L.; Sha, M.; Cui, C.; Zhenghong, Z. Effects of single high dose and multiple low dose cyclophosphamide abdominal injection on immunosuppressive model in mice. Chin. Anim. Husb. Vet. Med. 2021, 48, 3787–3794. [Google Scholar]
- Wang, C.; Gao, H.; Cai, E.; Zhang, L.; Zheng, X.; Zhang, S.; Sun, N.; Zhao, Y. Protective effects of Acanthopanax senticosus-Ligustrum lucidum combination on bone marrow suppression induced by chemotherapy in mice. Biomed. Pharmacother. 2019, 109, 2062–2069. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Ma, W.; Lu, Z.; Sun, C. Marine bacterial polysaccharide EPS11 inhibits cancer cell growth and metastasis via blocking cell adhesion and attenuating filiform structure formation. Mar. Drugs 2019, 17, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Meng, Q.; Qiao, H.; Jiang, H.; Sun, X. Role of the spleen in cyclophosphamide-induced hematosuppression and extramedullary hematopoiesis in mice. Arch. Med. Res. 2009, 40, 249–255. [Google Scholar] [CrossRef]
- Zheng, B.; Huang, Z.; Huang, Y.; Hong, L.; Li, J.; Wu, J. Predictive value of monocytes and lymphocytes for short-term neutrophil changes in chemotherapy-induced severe neutropenia in solid tumors. Support. Care Cancer 2020, 28, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Mones, J.V.; Soff, G. Management of thrombocytopenia in cancer patients. Cancer Treat. Res. 2019, 179, 139–150. [Google Scholar] [PubMed]
- Feng, L.; Huang, Q.; Huang, Z.; Li, H.; Qi, X.; Wang, Y.; Liu, Z.; Liu, X.; Lu, L. Optimized animal model of cyclophosphamide-induced bone marrow suppression. Basic Clin. Pharmacol. Toxicol. 2016, 119, 428–435. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Wang, H.; Wang, L.; Xiao, F.; Guo, Z.; Zhang, H. Surface phosphatidylserine is responsible for the internalization on microvesicles derived from hypoxia-induced human bone marrow mesenchymal stem cells into human endothelial cells. PLoS ONE 2016, 11, e0147360. [Google Scholar]
- Arab Tehrany, E.; Kahn, C.J.F.; Baravian, C.; Maherani, B.; Belhaj, N.; Wang, X.; Linder, M. Elaboration and characterization of nanoliposome made of soya; rapeseed and salmon lecithins: Application to cell culture. Colloids Surf. B Biointerfaces 2012, 95, 75–81. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Teng, S.; Zhang, L.; Teng, L.; Wang, D. Calf thymus polypeptide improved hematopoiesis via regulating colony- stimulating factors in BALB/c mice with hematopoietic dysfunction. Int. J. Biol. Macromol. 2020, 156, 204–216. [Google Scholar] [CrossRef]
- Lu, W.; Jia, D.; An, S.; Mu, M.; Qiao, X.; Liu, Y.; Li, X.; Wang, D. Calf spleen extractive injection protects mice against hematopoietic injury through signaling. Sci. Rep. 2017, 7, 8402. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Meng, M.; Geng, S.; Du, Z.; Zheng, Y.; Yao, J.; Li, Z.; Han, G.; Lin, H.; Du, G. The optimal dose of arsenic trioxide induced opposite efficacy in autophagy between K562 cells and their initiating cells to eradicate human myelogenous leukemia. J. Ethnopharmacol. 2017, 196, 29–38. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Shpakova, V.; Panteleev, M.; Kharazova, A. Multifaceted effects of arachidonic acid and interaction with cyclic nucleotides in human platelets. Thromb. Res. 2018, 171, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, H.K.A.; Fujiwara, Y.; Schlaeger, T.M.; Traver, D.; Orkin, S.H. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 2003, 101, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Engel, J.D.; Yamamoto, M. GATA1-related leukaemias. Nat. Rev. Cancer 2008, 8, 279–287. [Google Scholar] [CrossRef]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal absorption of fucoidan extracted from the brown deaweed, Cladosiphon okamuranus. Mar. Drugs 2015, 13, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption study of mozuku fucoidan in Japanese volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, E. Use of fluorescein isothiocyanate isomer I to study the mechanism of intestinal absorption of fucoidan sulfate in vivo and in vitro. Biopharm. Drug Dispos. 2018, 39, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Yang, X.; Zhang, Y.G.; Cai, E.B.; Zheng, X.M.; Zhao, Y.; Li, G.; Han, M.; Yang, L.M. Study on the changes of chemical constituents in different compatibilities of ginseng-prepared rehmannia root and their effects on bone marrow inhibition after chemotherapy. Chem. Pharm. Bull. 2020, 68, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Huilin, K.; Weiping, F.; Bo, L.; Yan, T.; Shuting, Z. The immunosuppression and immunoenhancement effect of cyclophosphamide on normal mice. Immunol. J. 2018, 34, 308–312. [Google Scholar]
- Yang, Y.; Hu, T.; Li, J.; Xin, M.; Zhao, X. Structural characterization and effect on leukopenia of fucoidan from Durvillaea antarctica. Carbohydr. Polym. 2021, 256, 117529. [Google Scholar] [CrossRef]
- Rhee, K.H.; Lee, K.H. Protective effects of fucoidan against γ-radiation-induced damage of blood cells. Arch. Pharm. Res. 2011, 34, 645–651. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Lipidomics: Techniques, applications, and outcomes related to biomedical sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storck, E.M.; Özbalci, C.; Eggert, U.S. Lipid cell biology: A focus on lipids in cell division. Annu. Rev. Biochem. 2018, 87, 839–869. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Black, A.; Kales, S.N.; Vattem, D.; Ruiz-Canela, M.; Sotos-Prieto, M. Metabolomics and microbiomes as potential tools to evaluate the effects of the mediterranean diet. Nutrients 2019, 11, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, X.; He, B.; Du, Y.; Sui, Z.; Rong, W.; Wang, X.; Li, Q.; Bi, K. The investigation of immunoprotective and sedative hypnotic effect of total polysaccharide from Suanzaoren decoction by serum metabonomics approach. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1086, 29–37. [Google Scholar] [CrossRef]
- Thomas, C.P.; Donnell, V.B.O. Oxidized phospholipid signaling in immune cells. Curr. Opin. Pharmacol. 2012, 12, 471–477. [Google Scholar] [CrossRef]
- Donnell, V.B.O.; Rossjohn, J.; Wakelam, M.J.O. Phospholipid signaling in innate immune cells. J. Clin. Investig. 2018, 128, 2670–2679. [Google Scholar] [CrossRef]
- Brodsky, R.A. Paroxysmal nocturnal hemoglobinuria. Blood 2014, 124, 2804–2811. [Google Scholar] [CrossRef]
- Zeharia, A.; Shaag, A.; Houtkooper, R.H.; Hindi, T.; de Lonlay, P.; Erez, G.; Hubert, L.; Saada, A.; de Keyzer, Y.; Eshel, G.; et al. Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am. J. Hum. Genet. 2008, 83, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Bijak, M.; Saluk-bijak, J. Flavonolignans inhibit the arachidonic acid pathway in blood platelets. BMC Complement. Altern. Med. 2017, 17, 396. [Google Scholar] [CrossRef] [Green Version]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Alpha-linolenic acid enhances the phagocytic and secretory functions of alternatively activated macrophages in part via changes to the oxylipin profile. Int. J. Biochem. Cell Biol. 2020, 119, 105662. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.N.; Leung, K.N. The immunomodulatory activity of Jacaric Acid, a conjugated linolenic acid isomer, on murine peritoneal macrophages. PLoS ONE 2015, 10, e0143684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kelly, O.J.; Ilich, J.Z. Synergism of α-linolenic acid, conjugated linoleic acid and calcium in decreasing adipocyte and increasing osteoblast cell growth. Lipids 2013, 48, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; Brash, A.R.; Murphy, R.C. The discovery and early structural studies of arachidonic acid. J. Lipid Res. 2016, 57, 1126–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, R.; Ghareghani, M.; Zibara, K.; Ardakani, M.T.; Jand, Y.; Azari, H. Alyssum homolocarpum seed oil (AHSO), containing natural alpha linolenic acid, stearic acid, myristic acid and β-sitosterol, increases proliferation and differentiation of neural stem cells in vitro. BMC Complement. Altern. Med. 2019, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Krombach, F.; Savolainen, K.M. Effect of linoleic acid, linoleic acid anilide, and arachidonic acid on the expression of adhesion molecules on human neutrophils. Arch. Toxicol. 1997, 71, 627–632. [Google Scholar]
- Mena, J.; Manosalva, C.; Ramirez, R.; Chandia, L.; Carroza, D.; Loaiza, A.; Burgos, R.A.; Hidalgo, M.A. Linoleic acid increases adhesion, chemotaxis, granule release, intracellular calcium mobilisation, MAPK phosphorylation and gene expression in bovine neutrophils. Vet. Immunol. Immunopathol. 2013, 151, 275–284. [Google Scholar] [CrossRef]
- Zhu, F.; Guan, Y.; Zhang, R. High-dose linoleic acid activated JAK2-STAT3 signaling pathway involved in cytokine production and lipogenesis in pancreatic exocrine cells. Curr. Mol. Med. 2016, 16, 668–676. [Google Scholar] [CrossRef]
- Thompson, B.; Valeri, C.R. Arachidonic acid metabolism by platelets of differing size. Br. J. Haematol. 1983, 53, 503–511. [Google Scholar]
- Liu, Y.T.; Li, X.Q.; Li, A.P.; Li, K.; Qin, X.M. UHPLC Q-Exactive MS-based spleen metabolomics and lipidomics to explore the effect mechanisms of Danggui Buxue Decoction in anemia mice. J. Pharm. Biomed. Anal. 2020, 185, 113234. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, J. Lipid metabolomic analysis of exosomes of osteonecrosis of the femoral head based on ultra performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2022, 40, 123–129. [Google Scholar]
- Anisimova, N.Y.; Ustyuzhanina, N.E.; Bilan, M.I.; Donenko, F.V.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Influence of modified fucoidan and related sulfated oligosaccharides on hematopoiesis in cyclophosphamide-induced mice. Mar. Drugs 2018, 16, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Kim, J.; Moon, C.; Kim, S.-H.; Hyun, J.W.; Park, J.W.; Shin, T. Radioprotective effects of fucoidan in mice treated with total body irradiation. Phyther. Res. 2008, 22, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Ai, C.; Wen, C.; Dong, X.; Sun, X.; Cao, C.; Zhang, X.; Zhu, B.; Song, S. Gut microbiota response to sulfated sea cucumber polysaccharides in a differential manner using an in vitro fermentation model. Food Res. Int. 2021, 148, 110562. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Zhang, R.; You, L.; Ma, Y.; Liao, L.; Pedisić, S. In vitro fermentation characteristics of polysaccharide from Sargassum fusiforme and its modulation effects on gut microbiota. Food Chem. Toxicol. 2021, 151, 112145. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xue, M.; Yang, J.; Pei, Z.; Zhang, N.; Qin, K.; Liang, H. Metabolic regulation mechanism of fucoidan via intestinal microecology in diseases. J. Sci. Food Agric. 2021, 101, 4456–4463. [Google Scholar] [CrossRef]
- Tao, Y.; Lei, W.; Jianda, J.; Qingchuan, Y.; Fei, Z.; Chen, X.; Jianzhong, X. The protective effect of two kinds of marine polysaccharides on the 60Co radiation-induced damage to mice. Radiat. Prot. 2019, 39, 242–248. [Google Scholar]







| Groups | First Day | Third Day | Fifth Day | Seventh Day |
|---|---|---|---|---|
| White Blood Cell Count (109/L, Mean ± SD) | ||||
| Control | 11.29 ± 2.83 | 12.21 ± 2.92 | 10.69 ± 3.81 | 14.55 ± 3.38 |
| Model | 4.46 ± 1.41 ** | 2.04 ± 1.16 ** | 3.85 ± 1.85 ** | 16.34 ± 3.58 |
| rhG-CSF | 4.48 ± 1.40 ** | 1.61 ± 0.52 ** | 11.03 ± 3.88 ## | 26.81 ± 4.31 **## |
| 1.8 mg/kg | 4.50 ± 1.41 ** | 1.91 ± 0.72 ** | 10.21 ± 4.45 ## | 22.44 ± 3.82 **## |
| 3.6 mg/kg | 4.50 ± 1.37 ** | 2.08 ± 0.78 ** | 8.39 ± 2.42 ## | 18.20 ± 2.95 |
| 7.2 mg/kg | 4.54 ± 1.46 ** | 1.72 ± 0.65 ** | 8.36 ± 3.40 ## | 15.99 ± 2.57 |
| Groups | Neutrophil Count (109/L, Mean ± SD) | |||
| Control | 4.21 ± 1.53 | 4.83 ± 1.57 | 3.76 ± 1.14 | 4.41 ± 1.19 |
| Model | 2.22 ± 0.98 ** | 0.47 ± 0.38 ** | 2.31 ± 1.38 ** | 15.25 ± 4.01 ** |
| rhG-CSF | 2.09 ± 0.83 ** | 0.57 ± 0.42 ** | 9.25 ± 3.76 **## | 24.11 ± 6.40 **# |
| 1.8 mg/kg | 2.03 ± 1.29 ** | 0.42 ± 0.27 ** | 7.86 ± 3.96 **## | 20.28 ± 8.93 **# |
| 3.6 mg/kg | 2.26 ± 0.97 ** | 0.35 ± 0.18 ** | 6.39 ± 2.38 ## | 16.65 ± 5.27 ** |
| 7.2 mg/kg | 2.43 ± 1.62 ** | 0.63 ± 0.66 ** | 6.43 ± 3.05 ## | 15.03 ± 4.33 ** |
| Groups | Platelet Count (109/L, Mean ± SD) | |||
| Control | 770.67 ± 58.75 | 758.07 ± 71.77 | 755.40 ± 135.06 | 758.20 ± 66.65 |
| Model | 769.33 ± 91.33 | 498.47 ± 70.46 ** | 359.27 ± 99.7 ** | 638.86 ± 179.33 |
| rhG-CSF | 813.93 ± 115.15 | 499.6 ± 91.21 ** | 360.33 ± 88.62 ** | 709.87 ± 117.90 |
| 1.8 mg/kg | 794.53 ± 58.65 | 445.93 ± 112.51 ** | 422.73 ± 69.20 ** | 850.71 ± 144.16 # |
| 3.6 mg/kg | 815.33 ± 116.58 | 467.8 ± 99.03 ** | 362.00 ± 106.44 ** | 771.80 ± 180.70 |
| 7.2 mg/kg | 858.40 ± 118.22 | 404.73 ± 124.02 ** | 347.93 ± 116.03 ** | 849.2 ± 256.08 # |
| No. | tR/min | m/z | HMDB ID | KEGG | Formula | Adduct | Identification | M vs. C | R vs. M | D vs. M |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11.98413 | 496.3399 | HMDB0061709 | - | C24H50NO7P | M+H, M+Na | 2-palmitoyl-sn-glycero-3-phosphocholine | ↓ | ↓ | |
| 2 | 12.46675 | 508.3765 | HMDB0013122 | C04230 | C26H54NO6P | M+H, M+Na | LysoPC (P-18:0) | ↓ | ↑ | |
| 3 | 13.00928 | 806.5689 | HMDB0007991 | C00157 | C46H80NO8P | M+H | PC (16:0/22:6) | ↓ | ||
| 4 | 13.68895 | 327.2332 | HMDB0002183 | C06429 | C22H32O2 | M-H | Docosahexaenoic acid | ↓ | ||
| 5 | 14.0069 | 810.6000 | HMDB0008464 | C00157 | C46H84NO8P | M+H | PC (20:4/18:0) | ↓ | ↓ | |
| 6 | 14.58425 | 465.3047 | HMDB0000653 | C18043 | C27H46O4S | M-H | Cholesterol sulfate | ↓ | ||
| 7 | 14.57367 | 760.5846 | HMDB0008295 | C00157 | C42H82NO8P | M+H | PC (20:1/14:0) | ↓ | ↑ | |
| 8 | 16.48282 | 768.5891 | HMDB0013407 | - | C44H82NO7P | M+H | PC (16:0/20:4) | ↓ | ||
| 9 | 16.5502 | 812.6139 | HMDB0008399 | C00157 | C46H86NO8P | M+H-H2O, M+H | PC (20:3/18:0) | ↓ | ↑ | |
| 10 | 16.7293 | 742.5735 | HMDB0008159 | C00157 | C42H80NO7P | M+H | PC (18:2/P-16:0) | ↓ | ||
| 11 | 16.8413 | 811.6675 | HMDB0012091 | C00550 | C45H93N2O6P | M+Na | SM (d18:0/22:0) | ↓ | ↑ | ↑ |
| 12 | 17.3122 | 806.569 | HMDB0008339 | C00157 | C46H80NO8P | M+H | PC (20:2/18:4) | ↓ | ||
| 13 | 17.42562 | 780.5533 | HMDB0008495 | C00157 | C44H78NO8P | M+H | PC (20:5/16:0) | ↓ | ↓ | |
| 14 | 17.44898 | 756.553 | HMDB0008199 | C00157 | C42H78NO8P | M+H | PC (18:3/16:0) | ↓ | ||
| 15 | 17.78938 | 627.5344 | HMDB0007171 | - | C41H72O5 | M+H-H2O | DG (18:0/20:4) | ↓ | ↓ | |
| 16 | 18.01642 | 806.5687 | HMDB0008212 | C00157 | C46H80NO8P | M+H | PC (18:3/20:3) | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.-P.; Yin, S.-N.; Chen, J.-P.; Geng, X.-C.; Liu, M.-F.; Li, H.-H.; Liu, M.; Liu, H.-B. Stimulating the Hematopoietic Effect of Simulated Digestive Product of Fucoidan from Sargassum fusiforme on Cyclophosphamide-Induced Hematopoietic Damage in Mice and Its Protective Mechanisms Based on Serum Lipidomics. Mar. Drugs 2022, 20, 201. https://doi.org/10.3390/md20030201
Ma W-P, Yin S-N, Chen J-P, Geng X-C, Liu M-F, Li H-H, Liu M, Liu H-B. Stimulating the Hematopoietic Effect of Simulated Digestive Product of Fucoidan from Sargassum fusiforme on Cyclophosphamide-Induced Hematopoietic Damage in Mice and Its Protective Mechanisms Based on Serum Lipidomics. Marine Drugs. 2022; 20(3):201. https://doi.org/10.3390/md20030201
Chicago/Turabian StyleMa, Wei-Ping, Shi-Ning Yin, Jia-Peng Chen, Xi-Cheng Geng, Ming-Fei Liu, Hai-Hua Li, Ming Liu, and Hong-Bing Liu. 2022. "Stimulating the Hematopoietic Effect of Simulated Digestive Product of Fucoidan from Sargassum fusiforme on Cyclophosphamide-Induced Hematopoietic Damage in Mice and Its Protective Mechanisms Based on Serum Lipidomics" Marine Drugs 20, no. 3: 201. https://doi.org/10.3390/md20030201