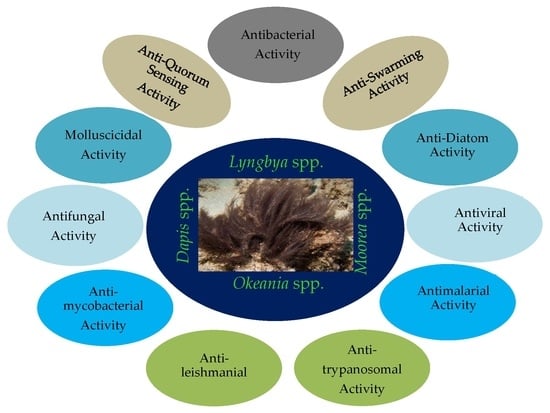
Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022
Abstract
:1. Introduction
2. Collected Species and Geographical Locations
3. Compounds with Antibacterial Activities
4. Compounds with Anti-Swarming and Anti-Quorum Sensing Activities
5. Compounds with Antifungal Activities
6. Compounds with Antiparasitic Activities
8. Compounds with Molluscicidal Anti-Diatoms Activities (Table 7)
| Compound | Source Organism | Collection Site | Targeted Organism | LC50/LC100/LD50/% of Inhibition | Reference |
|---|---|---|---|---|---|
| Tanikolide (34) | L. majuscula | Madagascar | B. glabrata | LD50 = 9.0 µg/mL | [62] |
| Barbamide (69) | L. majuscula | Curaçao | B. glabrata | LC100 = 10 µg/mL | [82] |
| Cyanolide A (70) | L. bouillonii | Papua New Guinea | B. glabrata | LC50 = 1.2 μM | [83] |
| Debromooscillatoxin G (71) | M. producens | Okinawa, Japan | N. amabilis | 30% at 10 μg/mL | [84] |
| Debromooscillatoxin I (72) | M. producens | Okinawa, Japan | N. amabilis | 30% at 10 μg/mL | [84] |
9. Summary
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, G.W.; Keating, C.L.; Newman, J.S. The golden anniversary of the silver bullet. JAMA 1993, 270, 1610–1611. [Google Scholar] [CrossRef]
- Kardos, N.; Demain, A.L. Penicillin: The medicine with the greatest impact on therapeutic outcomes. Appl. Microbiol. Biotechnol. 2011, 92, 677–687. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1334. [Google Scholar] [CrossRef] [Green Version]
- Freire-Moran, L.; Aronsson, B.; Manz, C.; Gyssens, I.C.; So, A.D.; Monnet, D.L.; Cars, O.; ECDC-EMA Working Group. Critical shortage of new antibiotics in development against multidrug-resistant bacteria-time to react is now. Drug Resist. Updat. 2011, 14, 118–124. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [Green Version]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 1, 36–42. [Google Scholar]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Antibiotic Resistance Threats in the United States; 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 25 October 2022).
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of infections due to MDR gram-negative bacteria. Front Med. 2019, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.-C. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front. Microbiol. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Poonawala, H.; Jain, Y. Drug-resistant tuberculosis: Is India ready for the challenge? BMJ Glob. Health 2018, 3, e000971. [Google Scholar] [CrossRef]
- Friedrich, M.J. Drug-resistant tuberculosis predicted to increase in high-burden countries. JAMA 2017, 318, 231. [Google Scholar] [CrossRef]
- Bloom, D.E.; Cadarette, D. Infectious disease threats in the twenty-first century: Strengthening the global response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.D.; Gao, S.-J. Global health concerns stirred by emerging viral infections. Med. Virol. 2020, 92, 399–400. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Pandemics throughout history. Front. Microbiol. 2021, 11, 3594. [Google Scholar] [CrossRef]
- Muñoz, L.S.; Garcia, M.A.; Gordon-Lipkin, E.; Parra, B.; Pardo, C.A. Emerging viral infections and their impact on the global burden of neurological disease. Semin. Neurol. 2018, 38, 163–175. [Google Scholar] [CrossRef]
- Bleibtreu, A.; Bertine, M.; Bertin, C.; Houhou-Fidouh, N.; Visseaux, B. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV). Med. Mal. Infect. 2020, 50, 243–251. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Ahmed, A.B.F.; Bhattacharjee, S.; Slama, P. Viral pandemics of the last four decades: Pathophysiology, health impacts and perspectives. Int. J. Environ. Res. Public Health 2020, 17, 9411. [Google Scholar] [CrossRef]
- Schaefer, T.J.; Panda, P.K.; Wolford, R.W. Dengue Fever; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Powell, J.R.; Human, M.-B. Viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018, 8, 1563–1565. [Google Scholar] [CrossRef]
- Singh, R.; Chauhan, N.; Kuddus, M. Exploring the therapeutic potential of marine-derived bioactive compounds against COVID-19. Environ. Sci. Pollut. Res. Int. 2021, 28, 52798–52809. [Google Scholar] [CrossRef]
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-billion to 3.5-billion-yearold) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef]
- Whitton, B.A.; Potts, M. The Ecology of Cyanobacteria: Their Diversity in Time and Space; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Nagle, D.G.; Paul, V.J. Production of secondary metabolites by filamentous tropical marine cyanobacteria: Ecological functions of the compounds. J. Phycol. 1999, 35, 1412–1421. [Google Scholar]
- Berry, J.P.; Gantar, M.; Perez, M.H.; Berry, G.; Noriega, F.G. Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar. Drugs 2008, 6, 117–146. [Google Scholar] [CrossRef]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Engene, N.; Rottacker, E.C.; Kas, J.; Byrum, T.; Gerwick, W.H.; Choi, H.; Ellisman, M.H. Moorea producens Gen. Nov., Sp. Nov. and Moorea bouillonii Comb. Nov., Tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 5, 1171–1178. [Google Scholar]
- Engene, N.; Tronholm, A.; Paul, V.J. Uncovering cryptic diversity of Lyngbya: The new tropical marine cyanobacterial genus Dapis (Oscillatoriales). J. Phycol. 2018, 54, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Jiří, K.; Eliška, Z.; Jan, Š.; Jiří, K.; Jason, W.; Brett, A.N.; Jaroslava, K. Polyphasic evaluation of Limnoraphis robusta, a water—Bloom forming cyanobacterium from Lake Atitlán, Guatemala, with a description of Limnoraphis gen. nov. J. Czech Phycol. Soc. 2013, 13, 39–52. [Google Scholar]
- Tronholm, A.; Engene, N. Moorena Gen. Nov., a valid name for “Moorea Engene & Al.” Nom. Inval. (Oscillatoriaceae, Cyanobacteria). Not. Algarum 2019, 122, 1–2. [Google Scholar]
- Mcgregor, G.B.; Sendall, B.C. Phylogeny and toxicology of Lyngbya wollei (Cyanobacteria, Oscillatoriales) from North-Eastern Australia, with description of Microseira gen. nov. J. Phycol. 2015, 51, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Paul, V.J.; Byrum, T.; Gerwick, W.H.; Thor, A.; Ellisman, M.H. Five chemically rich species of tropical marine cyanobacteria of the genus Okeania gen. nov. (Oscillatoriales, Cyanoprokaryota). J. Phycol. 2013, 49, 1095–1106. [Google Scholar] [CrossRef]
- Marine Pharmacology. Available online: https://www.marinepharmacology.org/ (accessed on 1 November 2022).
- Cardllina, J.H.C.; Moore, R.E.; Arnold, E.V.; Clardy, J. Structure and absolute configuration of malyngolide, an antibiotic from the marine blue-green alga Lyngbya majuscula Gomont. J. Org. Chem. 1979, 44, 4039–4042. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; McPhail, K.L.; Elbandy, M. Malyngamide 4, a new lipopeptide from the Red Sea marine cyanobacterium Moorea producens (Formerly Lyngbya majuscula). Phytochem. Lett. 2013, 6, 183–188. [Google Scholar] [CrossRef]
- Gekwick, W.H.; Reyes, S.; Alvarado, B. Two malyngamides from the Caribbean cyanobacterium Lyngbya majuscula. Phytochemistry 1987, 26, 1701–1704. [Google Scholar] [CrossRef]
- Luesch, H.; Pangilinan, R.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Pitipeptolides A and B, new cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 304–307. [Google Scholar] [CrossRef]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C-F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef] [Green Version]
- Montaser, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Pitiprolamide, a proline-rich dolastatin 16 analogue from the marine cyanobacterium Lyngbya majuscula from Guam. J. Nat. Prod. 2011, 74, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Zainuddin, E.N.; Jansen, R.; Nimtz, M.; Wray, V.; Preisitsch, M.; Lalk, M.; Mundt, S. Lyngbyazothrins A-D, antimicrobial cyclic undecapeptides from the cultured cyanobacterium Lyngbya sp. J. Nat. Prod. 2009, 72, 2080. [Google Scholar] [CrossRef] [Green Version]
- Levert, A.; Alvarin, R.; Bornancin, L.; Mansour, E.A.; Burja, A.M.; Genevie, A.; Bonnard, I.; Alonso, E.; Botana, L.; Banaigs, B. Structures and activities of tiahuramides A−C, cyclic depsipeptides from a Tahitian collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2018, 81, 1301–1310. [Google Scholar] [CrossRef]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef] [Green Version]
- Guttenplan, S.B.; Kearns, D.B. Regulation of flagellar motility during biofilm formation Sarah. FEMS Microbiol. Rev. 2013, 37, 849–871. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Tan, L.T. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 1810–1814. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Chan, K.P.; Chen, D.Y.K.; Tan, L.T. Lagunamide C, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2011, 72, 2369–2375. [Google Scholar] [CrossRef]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in bacterial quorum sensing: A biopharmaceutical perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Kwan, J.C.; Teplitski, M.; Gunasekera, S.P.; Paul, V.J.; Luesch, H. Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 463–466. [Google Scholar] [CrossRef] [Green Version]
- Dobretsov, S.; Teplitski, M.; Alagely, A.; Gunasekera, S.P.; Paul, V.J. Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environ. Microbiol. Rep. 2010, 2, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Meickle, T.; Ladwa, D.; Teplitski, M.; Paul, V.; Luesch, H. Lyngbyoic acid, a “Tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol. Biosyst. 2011, 7, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Matthew, S.; Chen, Q.Y.; Kwan, J.C.; Paul, V.J.; Luesch, H. Discovery and total synthesis of doscadenamide A: A quorum sensing signaling molecule from a marine cyanobacterium. Org. Lett. 2019, 21, 7274–7278. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Moore, R.E.; Mynderse, J.S.; Niemczura, W.P.; Todd, J.S. Structure of majusculamide C, a cyclic depsipeptide from Lyngbya majuscula. J. Org. Chem. 1984, 49, 236–241. [Google Scholar] [CrossRef]
- Mynderse, J.S.; Hunt, A.H.; Moore, R.E. 57-Normajusculamide C, a minor cyclic depsipeptide isolated from Lyngbya majuscula. J. Nat. Prod. 1988, 51, 1299–1301. [Google Scholar] [CrossRef]
- Meickle, T.; Matthew, S.; Ross, C.; Luesch, H.; Paul, V. Bioassay-guided isolation and identification of desacetylmicrocolin B from Lyngbya Cf. Polychroa. Planta Med. 2009, 75, 1427–1430. [Google Scholar] [CrossRef] [Green Version]
- Bonnard, I.; Rolland, M.; Francisco, C.; Banaigs, B. Total Structure and biological properties of laxaphycins A and B, cyclic lipopeptides from the marine cyanobacterium Lyngbya majuscula. Int. J. Pept. Res. Ther. 1997, 4, 289–292. [Google Scholar] [CrossRef]
- Singh, I.P.; Milligan, K.E.; Gerwick, W.H. Tanikolide, a toxic and antifungal lactone from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1999, 62, 1333–1335. [Google Scholar] [CrossRef]
- Milligan, K.E.; Marquez, B.L.; Williamson, R.T.; Gerwick, W.H. Lyngbyabellin B, a toxic and antifungal secondary metabolite from the marine cyanobacterium Lyngbya mojuscula. J. Nat. Prod. 2000, 63, 1440–1443. [Google Scholar] [CrossRef]
- Marquez, B.L.; Watts, K.S.; Yokochi, A.; Roberts, M.A.; Verdier-Pinard, P.; Jimenez, J.I.; Hamel, E.; Scheuer, P.J.; Gerwick, W.H. Structure and absolute stereochemistry of hectochlorin, a potent stimulator of actin assembly. J. Nat. Prod. 2002, 65, 866–871. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Ernst-Russell, M.A.; De Ropp, J.S.; Molinski, T.F. Lobocyclamides A-C, lipopeptides from a cryptic cyanobacterial mat containing Lyngbya confervoides. J. Org. Chem. 2002, 67, 8210–8215. [Google Scholar] [CrossRef]
- McPhail, K.L.; Correa, J.; Linington, R.G.; González, J.; Ortega-Barría, E.; Capson, T.L.; Gerwick, W.H. Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 984–988. [Google Scholar] [CrossRef] [Green Version]
- Balunas, M.J.; Linington, R.G.; Tidgewell, K.; Fenner, A.M.; Ureña, L.D.; Togna, G.D.; Kyle, D.E.; Gerwick, W.H. Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity. J. Nat. Prod. 2010, 73, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, M.; Tidgewell, K.; Capson, T.L.; Engene, N.; Almanza, A.; Schemies, J.; Jung, M.; Gerwick, W.H. Malyngolide dimer, a bioactive symmetric cyclodepside from the Panamanian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 709–711. [Google Scholar] [CrossRef] [Green Version]
- Nozaki, T.; Saito-nakano, Y.; Suenaga, K. Ikoamide, an antimalarial lipopeptide from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2020, 83, 481–488. [Google Scholar]
- Ozaki, K.; Iwasaki, A.; Sezawa, D.; Fujimura, H.; Nozaki, T.; Saito-nakano, Y.; Suenaga, K.; Teruya, T. Isolation and total synthesis of mabuniamide, a lipopeptide from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2019, 82, 2907–2915. [Google Scholar] [CrossRef]
- Shao, C.; Mou, X.; Cao, F.; Spadafora, C.; Glukhov, E.; Gerwick, L.; Wang, C.; Gerwick, W.H. Bastimolide B, an antimalarial 24-membered marine macrolide possessing a tert-butyl group. J. Nat. Prod. 2018, 81, 211–215. [Google Scholar] [CrossRef]
- Shao, C.L.; Linington, R.G.; Balunas, M.J.; Centeno, A.; Boudreau, P.; Zhang, C.; Engene, N.; Spadafora, C.; Mutka, T.S.; Kyle, D.E.; et al. Bastimolide A, a potent antimalarial polyhydroxy macrolide from the marine cyanobacterium Okeania hirsuta. J. Org. Chem. 2015, 80, 7849–7855. [Google Scholar] [CrossRef]
- Fathoni, I.; Petitbois, J.G.; Alarif, W.M.; Abdel-Lateff, A.; Al-lihaibi, S.S.; Yoshimura, E.; Nogata, Y.; Vairappan, C.S.; Sholikhah, E.N.; Okino, T. Bioactivities of lyngbyabellins from cyanobacteria of Moorea and Okeania genera. Molecules 2020, 5, 3986. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Lopez, D.; Vesely, B.A.; Della Togna, G.; Gerwick, W.H.; Kyle, D.E.; Linington, R.G. Almiramides A-C: Discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 2010, 53, 4187–4197. [Google Scholar] [CrossRef] [Green Version]
- Almaliti, J.; Malloy, K.L.; Glukhov, E.; Spadafora, C.; Gutiérrez, M.; Gerwick, W.H. Dudawalamides A-D, antiparasitic cyclic depsipeptides from the marine cyanobacterium Moorea producens. J. Nat. Prod. 2017, 80, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, N.; Iwasaki, A.; Jeelani, G.; Nozaki, T.; Suenaga, K. Iheyamides A-C, antitrypanosomal linear peptides isolated from a marine Dapis sp. cyanobacterium. J. Nat. Prod. 2020, 83, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Iwasaki, A.; Sumimoto, S.; Kanamori, Y.; Ohno, O.; Iwatsuki, M.; Ishiyama, A.; Hokari, R.; Otoguro, K.; Omura, S.; et al. Janadolide, a cyclic polyketide-peptide hybrid possessing a tert-butyl group from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2016, 79, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, R.; Iwasaki, A.; Ebihara, A.; Jeelani, G.; Nozaki, T.; Suenaga, K. Isolation and total synthesis of beru’amide, an antitrypanosomal polyketide from a marine cyanobacterium Okeania sp. Org. Lett. 2022, 24, 4710–4714. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.R.; Cardellina, J.H., 2nd; Fuller, R.W.; Weislow, O.S.; Kiser, R.F.; Snader, K.M.; Patterson, G.M.; Boyd, M.R. AIDS-antiviral sulfolipids from cyanobacteria (blue-green algae). J. Natl. Cancer Inst. 1989, 81, 1254–1258. [Google Scholar] [CrossRef]
- Reshef, V.; Mizrachi, E.; Maretzki, T.; Silberstein, C.; Loya, S.; Hizi, A.; Carmeli, S. New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J. Nat. Prod. 1997, 60, 1251–1260. [Google Scholar] [CrossRef]
- Loya, S.; Reshef, V.; Mizrachi, E.; Silberstein, C.; Rachamim, Y.; Carmeli, S.; Hizi, A. The inhibition of the reverse transcriptase of HIV-1 by the natural sulfoglycolipids from cyanobacteria: Contribution of different moieties to their high potency. J. Nat. Prod. 1998, 61, 891–895. [Google Scholar] [CrossRef]
- Orjala, J.; Gerwick, W.H. Barbamide, a chlorinated metabolite with molluscicidal activity from the Caribbean cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1996, 59, 427–430. [Google Scholar] [CrossRef]
- Pereira, A.R.; McCue, C.F.; Gerwick, W.H. Cyanolide A, a glycosidic macrolide with potent molluscicidal activity from the Papua New Guinea cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2010, 73, 217–220. [Google Scholar] [CrossRef]
- Iguchi, K.; Satake, M.; Nishio, Y.; Zhang, B.; Kawashima, K.; Uchida, H.; Nagai, H. Debromooscillatoxins G and I from the cyanobacterium Moorea Producens. Heterocycles 2021, 102, 1287–1293. [Google Scholar]



































| Species Name a | Number of Reported Compounds |
|---|---|
| Dapis sp. | 3 |
| Lyngbya sp. | 4 |
| Lyngbya bouillonii | 1 |
| Lyngbya confervoides | 3 |
| Lyngbya lagerheimii | 2 |
| Lyngbya majuscula | 35 |
| Lygnbya polychora | 2 |
| Moorea bouillonii | 4 |
| Moorea producens | 11 |
| Okeania sp. | 5 |
| Okeania hirsuta | 2 |
| Compound | Source Organism | Collection Site | Targeted Bacteria | MIC/Inhibition Zone/IC50 | Reference |
|---|---|---|---|---|---|
| Malyngolide (1) | L. majuscula | Hawaii, USA | M. smegmatis, S. pyogenes, S. aureus and B. subtilis | More active against M. smegmatis and S. pyogenes than S. aureus and B. subtilis | [40] |
| Lyngbic acid (2) | M. producens | Red Sea | M. tuberculosis H37Rv | 65% inhibition at 12.5 μg/mL | [41] |
| Lyngbic acid (2) | L. majuscula | Caribbean region | S. aureus and B. subtilis | Antibacterial activity | [42] |
| Malyngamide D acetate (3) | L. majuscula | Caribbean region | S. aureus | Slight activity | [42] |
| Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 25 mm at 100 µg 10 mm at 25 µg | [43] |
| Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 35818 | 15 mm at 100 µg 9 mm at 25 µg | [43] |
| Pitipeptolide B (5) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 30 mm at 100 µg 15 mm at 25 µg | [43] |
| Pitipeptolide B (5) | L. majuscula | Guam | M. tuberculosis ATCC 35818 | 15 mm at 100 µg 10 mm at 25 µg | [43] |
| Pitipeptolide A (4) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 28 mm at 100 µg 23 mm at 50 µg 9 mm at 10 µg | [44] |
| Pitipeptolide B (5) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 30 mm at 100 µg 24 mm at 50 µg 14 mm at 10 µg | [44] |
| Pitipeptolide C (6) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 26 mm at 100 µg 21 mm at 50 µg 18 mm at 10 µg | [44] |
| Pitipeptolide D (7) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 10 mm at 100 µg 0 mm at 50 µg 0 mm at 10 µg | [44] |
| Pitipeptolide E (8) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 21 mm at 100 µg 15 mm at 50 µg 0 mm at 10 µg | [44] |
| Pitipeptolide F (9) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 40 mm at 100 µg 30 mm at 50 µg 10 mm at 10 µg | [44] |
| Pitiprolamide (10) | L. majuscula | Guam | M. tuberculosis ATCC 25177 | 23 mm at 100 µg 13 mm at 50 µg 0 mm at 10 µg | [45] |
| Pitiprolamide (10) | L. majuscula | Guam | B. cereus ATCC 10987 | IC50 = 70 μM at 1 μM | [45] |
| Mixture of lyngbyazothrins A and B (14 and 15) | Lyngbya sp. | Germany (Culture) | M. flaVus SBUG 16 | 8 mm at 100 μg/disk | [46] |
| Mixture of lyngbyazothrins C (16) and D (17) | Lyngbya sp. | Germany (Culture) | B. subtilis SBUG 14 E. coli ATCC 11229 E. coli SBUG 13 P. aeruginosa ATCC 27853 S. marcescens SBUG 9 | 18 mm at 25 μg/disk 18 mm at 100 μg/disk 15 mm at 100 μg/disk 8 mm at 100 μg/disk 8 mm at 200 μg/disk | [46] |
| Tiahuramide A (18) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 27, 33, >50, 35 and 47 μM, respectively | [47] |
| Tiahuramide B (19) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 9.4, 8.5, 22, 12 and 29 μM, respectively | [47] |
| Tiahuramide C (20) | L. majuscula | French Polynesia | A. salmonicida (CIP 103209T strain), V. anguillarum (CIP 63.36T), S. baltica (CIP 105850T), E. coli (CIP 54.8) and M. luteus (CIP A270) | MIC = 6.7, 7.4, 16, 14 and 17 μM, respectively | [47] |
| Compound | Source Organism | Collection Site | Targeted Bacteria/Receptor | Anti-Swarming/Anti-Quorum Sensing | Reference |
|---|---|---|---|---|---|
| Lagunamide A (21) | L. majuscula | Singapore | P. aeruginosa PA01 | Anti-swarming effect: 62% at 100 ppm | [50,51] |
| Lagunamide B (22) | L. majuscula | Singapore | P. aeruginosa PA01 | Anti-swarming effect: 56% at 100 ppm | [50,51] |
| Lagunamide C (23) | L. majuscula | Singapore | P. aeruginosa PA01 | Anti-swarming effect: 49%, at 100 ppm | [50,51] |
| Malyngamide C (24) | L. majuscula | Florida, USA | 3-oxo-C12-HSL (N-3-oxo-dodecanoyl-L-homoserine lactone) signaling in a LasR-based quorum sensing (QS) reporter pSB1075 | QS inhibitor reduction in 3-oxo-C12-HSL signaling at 10, 100 and 1000 µM | [54] |
| 8-epi-Malyngamide C (25) | L. majuscula | Florida, USA | 3-oxo-C12-HSL (N-3-oxo-dodecanoyl-L-homoserine lactone) signaling in a LasR-based quorum sensing (QS) reporter pSB1075 | QS inhibitor reduction in 3-oxo-C12-HSL signaling at 10, 100 and 1000 µM | [54] |
| Malyngolide (1) | L. majuscula | Florida, USA | Production of violacein pigment by C. violaceum CV017 in the QS bioassay | QS inhibitor inhibition of violacein production with effective concentrations ranged from 0.07 to 0.22 mM; EC50 = 0.11 mM | [55] |
| Responses of lasR+PlasI-luxCDABE reporter pSB1075 in the presence of 14 µM of 3-oxo-C12-HSL | Inhibition of responses of the lasR+PlasI-luxCDABE reporter pSB1075 with concentrations ranging from 3.57 to 57; EC50 = 12.2 µM | [55] | |||
| Production of elastase by P. aeruginosa PAO1 (an extracellular enzyme regulated by 3-oxo-C12-HSL and LasR) | Significant reduction in elastase production; EC50 = 10.6 µM, at higher concentrations of MAL, elastase production was inhibited to the level observed in the QS mutant of P. aeruginosa JP2 | [55] | |||
| Lyngbyoic acid (26) | L. majuscula | Florida, USA | Four reporters based on different acylhomoserine lactone (AHL) receptors acylhomoserine lactone (AHL) receptors (LuxR, AhyR, TraR and LasR) | QS inhibitor, most effective inhibition against LasR reporter | [56] |
| Production of pyocyanin and elastase (LasB) both on the protein and transcript level in wild-type P. aeruginosa. | Reduction in the production of pyocyanin and elastase (LasB) and direct inhibition of LasB enzymatic activity; Ki = 5.4 mM | ||||
| Doscadenamide A (27) | L. bouillonii | Guam | 3-Oxo-C12-HSL-responsive reporter plasmid pSB1075, which encodes LasR and contains a light-producing luxCDABE cassette expressed in E. coli | QS agonist in a LasR-dependent manner and activation of 3-oxo-C12-HSL-responsive reporter plasmid pSB1075 | [57] |
| Production of QS pigment pyocyanin in wild-type P. aeruginosa | Increase pyocyanin production at 10 µM |
| Compound | Source Organism | Collection Site | Targeted Fungi | MIC/Inhibition Zone/LD50 | Reference |
|---|---|---|---|---|---|
| Majusculamide C (28) | L. majuscula | Marshall Islands | P. infestans and P. viticola | Growth inhibition | [58] |
| 57-Normajusculamide C (29) | L. majuscula | Marshall Islands | S. pastorianus | Antimycotic activity | [59] |
| Microcolin A (30) | L. polychroa | Marshall Islands | D. salina (SIO and EBGJ strains) | LD50 = >200 μg/mL | [60] |
| Microcolin B (31) | L. polychroa | Marshall Islands | D. salina (SIO and EBGJ strains) | LD50 = >200 μg/mL | [60] |
| Laxaphycin B (32) | L. majuscula | French Polynesia | C. albicans | Antifungal activity | [61] |
| Mixture of laxaphycins A (33) and B (32) | L. majuscula | French Polynesia | C. albicans | Laxaphycin B produces synergetic effect to the inactive laxaphycin A | |
| Tanikolide (34) | L. majuscula | Madagascar | C. albicans | 13 mm at 100 µg/disk | [62] |
| Lyngbyabellin B (35) | L. majuscula | Florida, USA | C. albicans (ATCC 14053) | 10.5 mm at 100 µg/disk | [63] |
| Hectochlorin (36) | L. majuscula | Jamaica | C. albicans (ATCC 14053) | 16 mm at 100 µg/disk 11 mm at 10 µg/disk | [64] |
| Lobocyclamide A (37) | L. confervoides | Southern Bahamas | C. albicans 96–489 (Fluconazole-resistant) | 7 mm at 150 µg/disk and MIC = 100 µg/mL | [65] |
| Lobocyclamide B (38) | L. confervoides | Southern Bahamas | C. albicans 96–489 (Fluconazole-resistant) | 8 mm at 150 µg/disk and MIC = 30–100 µg/mL | [65] |
| Lobocyclamide B (38) | L. confervoides | Southern Bahamas | C. glabrata | 6 mm at 150 µg/disk | [65] |
| Mixture of lobocyclamides A and B (37 and 38) | L. confervoides | Southern Bahamas | - | MIC = 10–30 µg/mL | [65] |
| Lobocyclamide C (39) | L. confervoides | Southern Bahamas | C. albicans 96–489 (Fluconazole-resistant) | 10 mm at 150 µg/disk | [65] |
| Lobocyclamides C (39) | L. confervoides | Southern Bahamas | C. glabrata | 8 mm at 150 µg/disk | [65] |
| Compound | Source Organism | Collection Site | Targeted Microbe/Parasite | IC50/% of Inhibition | Reference |
|---|---|---|---|---|---|
| Lagunamide A (21) | L. majuscula | Singapore | P. falciparum (NF54 strain) | IC50 = 0.19 μM | [50,51] |
| Lagunamide B (22) | L. majuscula | Singapore | P. falciparum (NF54 strain) | IC50 = 0.91 μM | [50,51] |
| Lagunamide C (23) | L. majuscula | Singapore | P. falciparum (NF54 strain) | IC50 = 0.29 μM | [50,51] |
| Carmabin A (40) | L. majuscula | Panama | P. falciparum (Indochina W2 strain) | IC50 = 4.3 µM | [66,67] |
| Dragomabin (41) | L. majuscula | Panama | P. falciparum (Indochina W2 strain) | IC50 = 6.0 µM | [66,67] |
| Dragonamide A (42) | L. majuscula | Panama | P. falciparum (Indochina W2 strain) | IC50 = 7.7 µM | [66,67] |
| Dragonamide A (42) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 6.5 μM | [67] |
| Malyngolide dimer (44) | L. majuscula | Panama | P. falciparum (W2 strain) | IC50 = 19 μM | [68] |
| Dragonamide E (53) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 5.1 μM | [67] |
| Herbamide B (54) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 5.9 μM | [67] |
| Almiramide B (55) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 2.4 μM | [74] |
| Almiramide C (56) | L. majuscula | Panama | L. donovani (LD-1S/MHOM/SD/00-strain 1S) | IC50 = 1.9 μM | [74] |
| Dudawalamide A (58) | M. producens | Papua New Guinea | P. falciparum | IC50 = 3.6 μM | [75] |
| Dudawalamide A (58) | M. producens | Papua New Guinea | T. cruzi | 12% inhibition at 10 μg/mL | [75] |
| Dudawalamide A (58) | M. producens | Papua New Guinea | L. donovani | IC50 = >10 μM | [75] |
| Dudawalamide B (59) | M. producens | Papua New Guinea | P. falciparum | IC50 = 10 μM | [75] |
| Dudawalamide B (59) | M. producens | Papua New Guinea | T. cruzi | 7% inhibition at 10 μg/mL | [75] |
| Dudawalamide B (59) | M. producens | Papua New Guinea | L. donovani | IC50 >10 μM | [75] |
| Dudawalamide C (60) | M. producens | Papua New Guinea | P. falciparum | IC50 = 3.5 μM | [75] |
| Dudawalamide D (61) | M. producens | Papua New Guinea | P. falciparum | IC50 = 8.0 μM | [75] |
| Dudawalamide D (61) | M. producens | Papua New Guinea | T. cruzi | 60% inhibition at 10 μg/mL | [75] |
| Dudawalamide D (61) | M. producens | Papua New Guinea | L. donovani | IC50 = 2.6 μM | [75] |
| Iheyamide A (62) | Dapis sp. | Okinawa, Japan | T. brucei rhodesiense T. bhurstuerusei brucei | IC50 = 1.5 μM IC50 = 1.5 μM | [76] |
| Janadolide (65) | Okeania sp. | Okinawa, Japan | T. brucei brucei | IC50 = 47 nM | [77] |
| Beru’amide (66) | Okeania sp. | Kagoshima, Japan | T. brucei rhodesiense | IC5 = 1.2 μM | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, D.T.A.; Mufti, S.J.; Badiab, A.A.; Shaala, L.A. Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022. Mar. Drugs 2022, 20, 768. https://doi.org/10.3390/md20120768
Youssef DTA, Mufti SJ, Badiab AA, Shaala LA. Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022. Marine Drugs. 2022; 20(12):768. https://doi.org/10.3390/md20120768
Chicago/Turabian StyleYoussef, Diaa T. A., Shatha J. Mufti, Abeer A. Badiab, and Lamiaa A. Shaala. 2022. "Anti-Infective Secondary Metabolites of the Marine Cyanobacterium Lyngbya Morphotype between 1979 and 2022" Marine Drugs 20, no. 12: 768. https://doi.org/10.3390/md20120768