Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage
Abstract
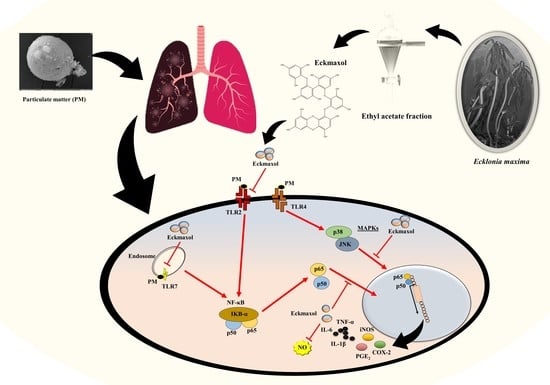
:1. Introduction
2. Results
2.1. Characterization of PM and Identification Eckmaxol
2.2. Effect of Eckmaxol on MH-S Lung Macrophages and PM-Stimulated Cell Viability and NO Production
2.3. Preventive Effect of Eckmaxol on Prostaglandin E2 (PGE-2) and Pro-Inflammatory Cytokine Production in PM-Induced MH-S Cells
2.4. Potential of Eckmaxol to Inhibit Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase-2 (COX-2) Gene and Protein Expression in PM Stimulated MH-S Lung Macrophages
2.5. Eckmaxol Suppresses the Pro-Inflammatory Cytokine Gene Expressions
2.6. Inhibitory Activity of Eckmaxol on the Expression of TLRs
2.7. Eckmaxol Inhibited the Nuclear Factor-κB (NF-κB) Nuclear Translocation and Mitogen-activated Protein Kinase (MAPK) Phosphorylation Induced via PM
3. Discussion
4. Materials and Methods
4.1. Chemicals and Regents
4.2. Isolation and Characterization of Eckmaxol
4.3. Morphological Analysis of PM
4.4. Cell Culture
4.4.1. MH-S Lung Macrophage Cell Culture
4.4.2. Cell Viability Assay and Dose-Range Determination for PM
4.4.3. Determination of Nitric Oxide (NO) Production
4.4.4. Evaluation of Pro-Inflammatory Cytokines and Prostaglandin E-2 (PGE-2) Production
4.5. Western Blotting
4.6. Evaluation of the NF-κB Nuclear Localization
4.7. Gene Expression Analysis
4.7.1. RNA Extraction and cDNA Synthesis
4.7.2. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Anastasio, C.; Martin, S.T. Atmospheric nanoparticles. Rev. Mineral. Geochem. 2001, 44, 293–349. [Google Scholar] [CrossRef]
- Yang, J.; Kim, Y.-K.; Kang, T.S.; Jee, Y.-K.; Kim, Y.-Y. Importance of indoor dust biological ultrafine particles in the pathogenesis of chronic inflammatory lung diseases. Environ. Health Toxicol. 2017, 32, e2017021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A., 3rd; Ezzati, M.; Dockery, D.W. Fine-particulate air pollution and life expectancy in the United States. N. Engl. J. Med. 2009, 360, 376–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudoniute, J.; Stasiulaitiene, I.; Kulvinskiene, I.; Bagdonas, E.; Garbaras, A.; Krugly, E.; Martuzevicius, D.; Bironaite, D.; Aldonyte, R. Pro-inflammatory effects of extracted urban fine particulate matter on human bronchial epithelial cells BEAS-2B. Environ. Sci. Pollut. Res. 2018, 25, 32277–32291. [Google Scholar] [CrossRef]
- Cao, X.-J.; Lei, F.-F.; Liu, H.; Luo, W.-Y.; Xiao, X.-H.; Li, Y.; Lu, J.-F.; Dong, Z.-B.; Chen, Q.-Z. Effects of Dust Storm Fine Particle-Inhalation on the Respiratory, Cardiovascular, Endocrine, Hematological, and Digestive Systems of Rats. Chin. Med. J. 2018, 131, 2482–2485. [Google Scholar] [CrossRef]
- Kroll, A.; Gietl, J.K.; Wiesmuller, G.A.; Gunsel, A.; Wohlleben, W.; Schnekenburger, J.; Klemm, O. In vitro toxicology of ambient particulate matter: Correlation of cellular effects with particle size and components. Environ. Toxicol. 2013, 28, 76–86. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.-Y.; Lee, H.G.; Je, J.-G.; Jee, Y.; Jeon, Y.-J. Sargassum horneri (Turner) inhibit urban particulate matter-induced inflammation in MH-S lung macrophages via blocking TLRs mediated NF-κB and MAPK activation. J. Ethnopharmacol. 2020, 249, 112363. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.K.; Lim, J.H.; Kim, Y.S.; Moon, W.K.; Choi, C.H.; Yoon, K.J.; Moon, S.H.; Lee, S.H.; Yeom, M.H. Composition for Alleviating Skin Inflammation Caused by Yellow Dust and Fine Particulate, Comprising Natural Plant Extract. U.S. Patent No. US20180207222A1, 26 July 2018. [Google Scholar]
- Jang, A.-S. Particulate Matter and Bronchial Asthma. Korean J. Med. 2015, 88, 150–155. [Google Scholar] [CrossRef]
- Akhtar, U.S.; McWhinney, R.D.; Rastogi, N.; Abbatt, J.P.; Evans, G.J.; Scott, J.A. Cytotoxic and proinflammatory effects of ambient and source-related particulate matter (PM) in relation to the production of reactive oxygen species (ROS) and cytokine adsorption by particles. Inhal. Toxicol. 2010, 22 (Suppl. S2), 37–47. [Google Scholar] [CrossRef]
- Gerlofs-Nijland, M.E.; Rummelhard, M.; Boere, A.J.; Leseman, D.L.; Duffin, R.; Schins, R.P.; Borm, P.J.; Sillanpaa, M.; Salonen, R.O.; Cassee, F.R. Particle induced toxicity in relation to transition metal and polycyclic aromatic hydrocarbon contents. Environ. Sci. Technol. 2009, 43, 4729–4736. [Google Scholar] [CrossRef]
- Gualtieri, M.; Ovrevik, J.; Holme, J.A.; Perrone, M.G.; Bolzacchini, E.; Schwarze, P.E.; Camatini, M. Differences in cytotoxicity versus pro-inflammatory potency of different PM fractions in human epithelial lung cells. Toxicol. In Vitro 2010, 24, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Schins, R.P.F.; Knaapen, A.M.; Weishaupt, C.; Winzer, A.; Borm, P.J.A. Cytotoxic and Inflammatory Effects of Coarse and Fine Particulate Matter in Macrophages and Epithelial Cells. Ann. Occup. Hyg. 2002, 46 (Suppl. S1), 203–206. [Google Scholar]
- Hoek, J.B.; Pastorino, J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol 2002, 27, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Borroni, E.M.; Mantovani, A.; Locati, M.; Bonecchi, R. Chemokine receptors intracellular trafficking. Pharmacol. Ther. 2010, 127, 1–8. [Google Scholar] [CrossRef]
- Dorschmann, P.; Bittkau, K.S.; Neupane, S.; Roider, J.; Alban, S.; Klettner, A. Effects of Fucoidans from Five Different Brown Algae on Oxidative Stress and VEGF Interference in Ocular Cells. Mar. Drugs 2019, 17, 258. [Google Scholar] [CrossRef] [Green Version]
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. Macromol. 2020, 151, 412–420. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Huang, C.; Zhao, J.; Lin, J.; Zhou, X.; Naman, C.B.; Wang, N.; Gerwick, W.H.; Wang, Q.; et al. Eckmaxol, a Phlorotannin Extracted from Ecklonia maxima, Produces Anti-beta-amyloid Oligomer Neuroprotective Effects Possibly via Directly Acting on Glycogen Synthase Kinase 3beta. ACS Chem. Neurosci. 2018, 9, 1349–1356. [Google Scholar] [CrossRef]
- Lopes, G.; Andrade, P.B.; Valentao, P. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2016, 22, 56. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Heo, S.-J.; Park, E.-J.; Lee, K.-W.; Jeon, Y.-J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Walsh, N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006, 44, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Braden, K.W.; Blanton, J.R., Jr.; Allen, V.G.; Pond, K.R.; Miller, M.F. Ascophyllum nodosum supplementation: A preharvest intervention for reducing Escherichia coli O157:H7 and Salmonella spp. in feedlot steers. J. Food Prot. 2004, 67, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.J.; Yoon, K.D.; Min, S.Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef] [Green Version]
- Le, Q.-T.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Wang, L.; Je, J.-G.; Kim, H.-S.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y.-J. Anti-Melanogenesis and Photoprotective Effects of Ecklonia maxima Extract Containing Dieckol and Eckmaxol. Mar. Drugs 2022, 20, 557. [Google Scholar] [CrossRef]
- Kim, H.-S.; Je, J.-G.; An, H.; Baek, K.; Lee, J.M.; Yim, M.-J.; Ko, S.-C.; Kim, J.-Y.; Oh, G.-W.; Kang, M.-C.; et al. Isolation and Characterization of Efficient Active Compounds Using High-Performance Centrifugal Partition Chromatography (CPC) from Anti-Inflammatory Activity Fraction of Ecklonia maxima in South Africa. Mar. Drugs 2022, 20, 471. [Google Scholar] [CrossRef]
- Mori, I.; Sun, Z.; Ukachi, M.; Nagano, K.; McLeod, C.W.; Cox, A.G.; Nishikawa, M. Development and certification of the new NIES CRM 28: Urban aerosols for the determination of multielements. Anal. Bioanal. Chem. 2008, 391, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J.D. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae1. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wu, J.; Wang, S.; Shu, H.; Zhang, M.; Liu, K.; Liu, K. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int. J. Biol. Macromol. 2019, 129, 377–385. [Google Scholar] [CrossRef]
- He, M.; Ichinose, T.; Ren, Y.; Song, Y.; Yoshida, Y.; Arashidani, K.; Yoshida, S.; Nishikawa, M.; Takano, H.; Sun, G. PM2.5-rich dust collected from the air in Fukuoka, Kyushu, Japan, can exacerbate murine lung eosinophilia. Inhal. Toxicol. 2015, 27, 287–299. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Hartung, T.; Huber, C.; Vollmar, A.M. Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-κB pathway. J. Hepatol. 2003, 38, 289–297. [Google Scholar] [CrossRef]
- Salter, D. Connective Tissue Responses to Mechanical Stresses. In Rheumatology; Elsevier-Health Sciences Division: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Akira, S. Toll-like Receptors and Innate Immunity. In Advances in Immunology; Dixon, F.J., Ed.; Academic Press: Cambridge, MA, USA, 2001; Volume 78, pp. 1–56. [Google Scholar]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Ahn, G.; Kim, H.-J.; Fu, X.; Jee, Y.; Jeon, Y.-J. Ethanol extract separated from Sargassum horneri (Turner) abate LPS-induced inflammation in RAW 264.7 macrophages. Fish. Aquat. Sci. 2019, 22, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Yi, M.; Ding, L.; He, S.; Yan, X. Isolation and Purification of a Neuroprotective Phlorotannin from the Marine Algae Ecklonia maxima by Size Exclusion and High-Speed Counter-Current Chromatography. Mar. Drugs 2019, 17, 212. [Google Scholar] [CrossRef] [Green Version]
- Jayawardena, T.U.; Sanjeewa, K.; Lee, H.-G.; Nagahawatta, D.; Yang, H.-W.; Kang, M.-C.; Jeon, Y.-J. Particulate Matter-Induced Inflammation/Oxidative Stress in Macrophages: Fucosterol from Padina boryana as a Potent Protector, Activated via NF-κB/MAPK Pathways and Nrf2/HO-1 Involvement. Mar. Drugs 2020, 18, 628. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Jayawardena, T.U.; Kim, H.-S.; Lee, W.W.; Vaas, A.; De Silva, H.; Abayaweera, G.; Nanayakkara, C.; Abeytunga, D.; Lee, D.-S. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahawatta, D.P.; Kim, H.-S.; Jee, Y.-H.; Jayawardena, T.U.; Ahn, G.; Namgung, J.; Yeo, I.-K.; Sanjeewa, K.K.A.; Jeon, Y.-J. Sargachromenol Isolated from Sargassum horneri Inhibits Particulate Matter-Induced Inflammation in Macrophages through Toll-like Receptor-Mediated Cell Signaling Pathways. Mar. Drugs 2022, 20, 28. [Google Scholar] [CrossRef] [PubMed]







| Gene | Primer | Sequence |
|---|---|---|
| GAPDH | Sense | 5′-AAGGGTCATCATCTCTGCCC-3′ |
| Antisense | 5′-GTGATGGCATGGACTGTGGT-3′ | |
| iNOS | Sense | 5′-ATGTCCGAAGCAAACATCAC-3′ |
| Antisense | 5′-TAATGTCCAGGAAGTAGGTG-3′ | |
| COX-2 | Sense | 5′-CAGCAAATCCTTGCTGTTCC-3′ |
| Antisense | 5′-TGGGCAAAGAATGCAAACATC-3′ | |
| IL-6 | Sense | 5′-GTACTCCAGAAGACCAGAGG-3′ |
| Antisense | 5′-TGCTGGTGACAACCACGGCC-3′ | |
| IL-1β | Sense | 5′-CAGGATGAGGACATGAGCACC-3′ |
| Antisense | 5′-CTCTGCAGACTCAAACTCCAC-3′ | |
| TNF-α | Sense | 5′-TTGACCTCAGCGCTGAGTTG-3′ |
| Antisense | 5′-CCTGTAGCCCACGTCGTAGC-3′ | |
| TLR-2 | Sense | 5′-CAGCTGGAGAACTCTGACCC-3′ |
| Antisense | 5′-CAAAGAGCCTGAAGTGGGAG-3′ | |
| TLR-4 | Sense | 5′-CAACATCATCCAGGAAGGC-3 |
| Antisense | 5′-GAAGGCGATACAATTCCACC-3′ | |
| TLR-7 | Sense | 5′-TTCCTTCCGTAGGCTGAACC-3′ |
| Antisense | 5′-GTAAGCTGGATGGCAGATCC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Lee, H.-G.; Heo, M.-S.; Jeon, Y.-J. Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage. Mar. Drugs 2022, 20, 766. https://doi.org/10.3390/md20120766
Nagahawatta DP, Liyanage NM, Jayawardhana HHACK, Jayawardena TU, Lee H-G, Heo M-S, Jeon Y-J. Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage. Marine Drugs. 2022; 20(12):766. https://doi.org/10.3390/md20120766
Chicago/Turabian StyleNagahawatta, D. P., N. M. Liyanage, H. H. A. C. K. Jayawardhana, Thilina U. Jayawardena, Hyo-Geun Lee, Moon-Soo Heo, and You-Jin Jeon. 2022. "Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage" Marine Drugs 20, no. 12: 766. https://doi.org/10.3390/md20120766