Discovery of Novel Pimprinine and Streptochlorin Derivatives as Potential Antifungal Agents
Abstract
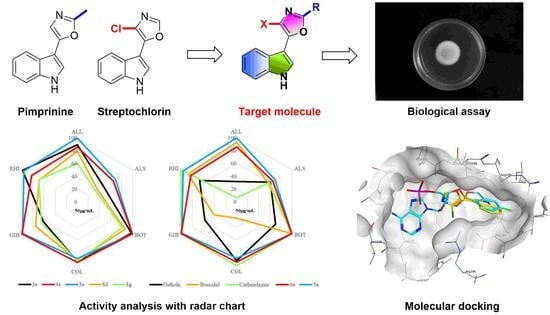
:1. Introduction
2. Results and Discussion
2.1. Synthetic Chemistry
2.2. Antifungal Activity and Structure–Activity Relationships (SAR)
2.3. Molecular Modeling
3. Materials and Methods
3.1. Chemistry
3.1.1. Preparation of 1-(1H-indol-3-yl)ethan-1-one (2)
3.1.2. Preparation of 2-substituted-5-(1H-indol-3-yl)-oxazole (3)
3.1.3. General Procedure for the Synthesis of 2-substituted-4-halogen-5-(1H-indol-3-yl)oxazole (4 or 5)
3.1.4. Synthesis of Substituted 5-(1H-indol-3-yl)-2-methyloxazoles (8–10)
3.2. Biological Assays
3.3. Molecular Modeling Strategy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aaRS | aminoacyl-tRNA synthetase |
| AMP | adenosine monophosphate |
| DME | 1,2-dimethoxyethane |
| DMF | N,N-dimethylformamide |
| DMSO | dimethylsulfoxide |
| HRMS | high-resolution mass spectra |
| LeuRS | leucyl-tRNA synthetase |
| m.p. | melting point |
| NBS | N-bromosuccinimide |
| NCS | N-chlorosuccinimide |
| NMR | nuclear magnetic resonance |
| THF | tetrahydrofuran |
| TLC | thin layer chromatography |
| tLeuRS | Thermus thermophiles leucyl-tRNA synthetase |
| v/v | ratio by volume |
References
- De Rop, A.S.; Rombaut, J.; Willems, T.; De Graeve, M.; Vanhaecke, L.; Hulpiau, P.; De Maeseneire, S.L.; De Mol, M.L.; Soetaert, W.K. Novel Alkaloids from Marine Actinobacteria: Discovery and Characterization. Mar. Drugs 2022, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; El-Sayed Hamed, A.N.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal potential of marine natural products. Eur. J. Med. Chem. 2017, 126, 631–651. [Google Scholar] [CrossRef]
- Dai, J.K.; Dan, W.J.; Wan, J.B. Natural and synthetic beta-carboline as a privileged antifungal scaffolds. Eur. J. Med. Chem. 2022, 229, 114057. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, B.S.; Taylor, W.I.; Bhate, D.S.; Karmarkar, S.S. The structure and synthesis of pimprinine. Tetrahedron 1963, 19, 1437–1439. [Google Scholar] [CrossRef]
- Lever, J.; Kreuder, F.; Henry, J.; Hung, A.; Allard, P.M.; Brkljaca, R.; Rix, C.; Taki, A.C.; Gasser, R.B.; Kaslin, J.; et al. Targeted Isolation of Antibiotic Brominated Alkaloids from the Marine Sponge Pseudoceratina durissima Using Virtual Screening and Molecular Networking. Mar. Drugs 2022, 20, 554. [Google Scholar] [CrossRef]
- Watanabe, H.; Amano, S.; Yoshida, J.; Takase, Y.; Miyadoh, S.; Sasaki, T.; Hatsu, M.; Takeuchi, Y.; Kodama, Y. A new antibiotic SF2583A, 4-chloro-5-(3′-indolyl) oxazole, produced by Streptomyces. Meiji Seika Kenkyu Nenpo 1988, 27, 55–62. [Google Scholar]
- Nishida, A.; Fuwa, M.; Naruto, S.; Sugano, Y.; Saito, H.; Nakagawa, M. Solid-phase synthesis of 5-(3-indolyl)oxazoles that inhibit lipid peroxidation. Tetrahedron. Lett. 2000, 41, 4791–4794. [Google Scholar] [CrossRef]
- Takahashi, S.; Matsunaga, T.; Hasegawa, C.; Saito, H.; Fujita, D.; Kiuchi, F.; Tsuda, Y. Martefragin A, a novel indole alkaloid isolated from red alga, inhibits lipid peroxidation. Chem. Pharm. Bull. 1998, 46, 1527–1529. [Google Scholar] [CrossRef] [Green Version]
- Kwak, T.W.; Shin, H.J.; Jeong, Y.I.; Han, M.E.; Oh, S.O.; Kim, H.J.; Kim, D.H.; Kang, D.H. Anticancer activity of streptochlorin, a novel antineoplastic agent, in cholangiocarcinoma. Drug Des. Dev. Ther. 2015, 9, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wan, Z.; Yang, S.; Liu, F.; Yang, X.; Wu, Z.; Zhang, Y.; Wang, K.; Fang, W. Two new dipimprinine alkaloids from soil-derived Streptomyces sp. 44414B. J. Antibiot. 2021, 74, 474–476. [Google Scholar] [CrossRef]
- Wei, Y.; Fang, W.; Wan, Z.; Wang, K.; Yang, Q.; Cai, X.; Shi, L.; Yang, Z. Antiviral effects against EV71 of pimprinine and its derivatives isolated from Streptomyces sp. Virol. J. 2014, 11, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Li, R.; Li, Y.; Li, S.; Yu, J.; Zhao, B.; Liao, A.; Wang, Y.; Wang, Z.; Lu, A.; et al. Discovery of Pimprinine Alkaloids as Novel Agents against a Plant Virus. J. Agric. Food Chem. 2019, 67, 1795–1806. [Google Scholar] [CrossRef]
- Choi, I.K.; Shin, H.J.; Lee, H.S.; Kwon, H.J. Streptochlorin, a marine natural product, inhibits NF-κB activation and suppresses angiogenesis in vitro. J. Microbiol. Biotechnol. 2007, 17, 1338–1343. [Google Scholar]
- Kroiss, J.; Kaltenpoth, M.; Schneider, B.; Schwinger, M.G.; Hertweck, C.; Maddula, R.K.; Strohm, E.; Svatos, A. Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 2010, 6, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Shin, H.J.; Kim, G.Y.; Kwon, T.K.; Nam, T.J.; Kim, S.K.; Cheong, J.; Choi, I.W.; Choi, Y.H. Induction of apoptosis by streptochlorin isolated from Streptomyces sp. in human leukemic U937 cells. Toxicol. Vitr. 2008, 22, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Mulholland, N.; Seville, A.; Hough, G.; Smith, N.; Dong, H.Q.; Zhang, W.H.; Gu, Y.C. First discovery of pimprinine derivatives and analogs as novel potential herbicidal, insecticidal and nematicidal agents. Tetrahedron 2021, 79, 131835. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Chen, Q.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y.C.; Yang, G.F.; Clough, J. Synthesis and fungicidal activity of novel pimprinine analogues. Eur. J. Med. Chem. 2012, 53, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y.C.; Chen, Q.; Yang, G.F.; Clough, J. Synthesis and antifungal activity of 3-(1,3,4-oxadiazol-5-yl)-indoles and 3-(1,3,4-oxadiazol-5-yl)methyl-indoles. Eur. J. Med. Chem. 2013, 63, 22–32. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Chen, Q.; Xie, C.H.; Mulholland, N.; Turner, S.; Irwin, D.; Gu, Y.C.; Yang, G.F.; Clough, J. Synthesis and antifungal activity of novel streptochlorin analogues. Eur. J. Med. Chem. 2015, 92, 776–783. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Jia, C.Y.; Gu, Y.C.; Mulholland, N.; Turner, S.; Beattie, D.; Zhang, W.H.; Yang, G.F.; Clough, J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017, 126, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.Y.; Xu, L.Y.; Yu, X.; Ding, Y.B.; Jin, B.; Zhang, M.Z.; Zhang, W.H.; Yang, G.F. An efficient synthesis and antifungal evaluation of natural product streptochlorin and its analogues. Fitoterapia 2018, 125, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.L.; Zhu, Y.; Liu, J.R.; Guo, S.K.; Gu, Y.C.; Han, X.Y.; Dong, H.Q.; Sun, Q.; Zhang, W.H.; Zhang, M.Z. Diversity-oriented synthesis and antifungal activities of novel pimprinine derivative bearing a 1,3,4-oxadiazole-5-thioether moiety. Mol. Divers. 2021, 25, 205–221. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, D.C.; Liu, C.; Song, Z.L.; Liu, J.R.; Guo, S.K.; Tan, J.Y.; Qiu, R.L.; Jin, B.; Zhang, H.F.; et al. Streptochlorin analogues as potential antifungal agents: Design, synthesis, antifungal activity and molecular docking study. Bioorg. Med. Chem. 2021, 35, 116073. [Google Scholar] [CrossRef]
- Xiang, J.C.; Wang, J.G.; Wang, M.; Meng, X.G.; Wu, A.X. One-pot total synthesis: The first total synthesis of chiral alkaloid pimprinol A and the facile construction of its natural congeners from amino acids. Tetrahedron 2014, 70, 7470–7475. [Google Scholar] [CrossRef]
- Naik, S.R.; Harindran, J.; Varde, A.B. Pimprinine, an extracellular alkaloid produced by Streptomyces CDRIL-312: Fermentation, isolation and pharmacological activity. J. Biotechnol. 2001, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Ogawa, K.; Iinuma, H.; Suda, H.; Ukita, K. Monoamine oxidase inhibitors isolated from fermented broths. J. Antibiot. 1973, 26, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, F.L.; Mao, W.M.; Yaremchuk, A.; Tukalo, M.; Crepin, T.; Zhou, H.C.; Zhang, Y.K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, O.; Neder, A.D.; Dias, A.K.; Cruz, R.P.; Aquino, L.B. Acylation of indole under Friedel-Crafts conditions-an improved method to obtain 3-acylindoles regioselectively. Org. Lett. 2001, 3, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Ng, T.B.; Zhang, J.; Zhou, M.; Song, F.; Lu, F.; Liu, Y. Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides 2007, 28, 553–559. [Google Scholar] [CrossRef] [PubMed]







| No. | Amino Acid | R1 = | Yield |
|---|---|---|---|
| 3a | Glycine | H | 51% |
| 3b (Pimprinine) | Alanine | Me | 53% |
| 3c (Pimprinethine) | 2-Aminobutyric acid | Et | 49% |
| 3d (Labradorins 1) | Leucine |  | 48% |
| 3e | Cyclohexylglycine |  | 53% |
| 3f | Phenylglycine |  | 48% |
| 3g | Methionine |  | 40% |
| No. | R1 = | X = | Yield | No. | R1 = | X = | Yield |
|---|---|---|---|---|---|---|---|
| 4a | H | Cl | 58% | 5a | H | Br | 62% |
| 4b |  | Cl | 65% | 5b |  | Br | 76% |
| 4c |  | Cl | 60% | 5c |  | Br | 65% |
| 4d |  | Cl | 76% | 5d |  | Br | 74% |
| 4e |  | Cl | 62% | 5e |  | Br | 77% |
| 4f |  | Cl | 57% | 5f |  | Br | 70% |
| 4g |  | Cl | / a | 5g |  | Br | 50% |
| No. | R2 = | R3 = | X = | Yield | No. | R2 = | R3 = | X = | Yield |
|---|---|---|---|---|---|---|---|---|---|
| 8a | 5-F | Me | H | 35% | 9e | 6-F | Et | Cl | 52% |
| 8b | 5-Cl | Me | H | 50% | 9f | 6-Cl | Me | Cl | 33% |
| 8c | 5-Br | Me | H | 43% | 9g | 6-Cl | Et | Cl | 74% |
| 8d | 4-Me | Me | H | 32% | 9h | 6-Br | Et | Cl | 60% |
| 8e | 6-F | Me | H | 61% | 9i | 5-Me | Et | Cl | 41% |
| 8f | 6-Cl | Me | H | 66% | 10a | 5-F | Me | Br | 41% |
| 8g | 5-Me | Me | H | 39% | 10b | 5-Cl | Me | Br | 43% |
| 8h | 6-F | Et | H | 59% | 10c | 5-Br | Me | Br | 56% |
| 8i | 6-Cl | Et | H | 58% | 10d | 4-Me | Me | Br | 49% |
| 8j | 6-Br | Et | H | 36% | 10e | 6-F | Me | Br | 66% |
| 8k | 5-Me | Et | H | 45% | 10f | 6-F | Et | Br | 37% |
| 9a | 5-F | Me | Cl | 30% | 10g | 6-Cl | Me | Br | 68% |
| 9b | 5-Cl | Me | Cl | 35% | 10h | 6-Cl | Et | Br | 54% |
| 9c | 5-Br | Me | Cl | 42% | 10i | 6-Br | Et | Br | 70% |
| 9d | 6-F | Me | Cl | 39% |
 | ||||||||
| No. | R = | X = | Growth Inhibition (%) | |||||
| ALL a | ALS | BOT | COL | GIB | RHI | |||
| 3a | H | H | 89.1b | 47.8 | 97.7 | 93.3 | 61.0 | 98.3 |
| 3b (Pimprinine) |  | H | 62.6 | 50.0 | 50.0 | 50.3 | 59.3 | 66.7 |
| 3c (Pimprinethine) |  | H | 53.2 | 46.7 | 85.7 | 52.2 | 59.7 | 73.3 |
| 3d (Labradorins 1) |  | H | 29.3 | 20.7 | 44.3 | 46.0 | 47.4 | 61.3 |
| 3e |  | H | 0.0 | 16.9 | 7.1 | 52.7 | 4.5 | 25.3 |
| 3f |  | H | 0.0 | 15.1 | 15.7 | 41.6 | 13.6 | 17.3 |
| 3g |  | H | 23.8 | 38.3 | 40.0 | 18.0 | 15.7 | 68.0 |
| 4a (Streptochlorin) | H | Cl | 85.5 | 65.4 | 99.9 | 94.6 | 99.9 | 82.4 |
| 4b |  | Cl | 77.3 | 46.1 | 70.0 | 62.7 | 71.5 | 85.0 |
| 4c |  | Cl | 58.2 | 27.7 | 57.1 | 77.6 | 50.3 | 67.3 |
| 4d |  | Cl | 50.8 | 33.8 | 45.7 | 64.5 | 51.5 | 72.0 |
| 4e |  | Cl | 0.0 | 23.1 | 10.0 | 54.7 | 13.8 | 26.7 |
| 4f |  | Cl | 29.8 | 20.0 | 30.0 | 45.6 | 22.7 | 30.7 |
| 5a | H | Br | 99.9 | 69.0 | 99.9 | 88.3 | 98.7 | 96.1 |
| 5b |  | Br | 68.0 | 25.5 | 71.4 | 66.0 | 60.0 | 82.7 |
| 5c |  | Br | 62.2 | 41.6 | 65.7 | 67.2 | 56.5 | 61.3 |
| 5d |  | Br | 50.4 | 46.5 | 72.9 | 60.5 | 54.6 | 81.3 |
| 5e |  | Br | 0.0 | 27.7 | 8.6 | 50.7 | 17.1 | 28.0 |
| 5f |  | Br | 39.7 | 24.6 | 25.7 | 47.1 | 9.1 | 40.0 |
| 5g |  | Br | 11.1 | 10.8 | 14.3 | 47.3 | 7.6 | 49.3 |
| Osthole | / | / | 31.3 | 61.2 | 70.4 | 92.3 | 57.0 | 66.5 |
| Boscalid | / | / | 92.8 | 57.6 | 99.9 | 25.5 | 40.9 | 87.3 |
| Carbendazim | / | / | 6.4 | 59.6 | 99.9 | 99.9 | 99.9 | 99.9 |
 | |||||||||
| No. | R2 = | R3 = | X = | Growth inhibition (%) | |||||
| ALL a | ALS | BOT | COL | GIB | RHI | ||||
| 8a | 5-F | Me | H | 53.2 b | 24.4 | 12.1 | 24.1 | 38.3 | 47.5 |
| 8b | 5-Cl | Me | H | 44.9 | 12.7 | 25.8 | 23.4 | 40.6 | 45.1 |
| 8c | 5-Br | Me | H | 37.8 | 24.8 | 35.9 | 90.1 | 37.5 | 46.4 |
| 8d | 4-Me | Me | H | 79.0 | 37.0 | 86.0 | 91.2 | 75.3 | 68.5 |
| 8e | 6-F | Me | H | 46.0 | 18.5 | 34.6 | 41.4 | 37.5 | 61.7 |
| 8f | 6-Cl | Me | H | 43.1 | 21.7 | 31.8 | 42.4 | 43.9 | 75.5 |
| 8g | 5-Me | Me | H | 60.3 | 33.9 | 80.9 | 92.8 | 64.7 | 69.0 |
| 8h | 6-F | Et | H | 57.1 | 41.5 | 42.6 | 68.5 | 55.8 | 79.2 |
| 8i | 6-Cl | Et | H | 28.7 | 11.4 | 26.7 | 32.6 | 43.1 | 61.2 |
| 8j | 6-Br | Et | H | 28.2 | 17.2 | 21.4 | 27.5 | 35.1 | 62.5 |
| 8k | 5-Me | Et | H | 39.7 | 14.2 | 65.5 | 87.2 | 55.9 | 73.4 |
| 9a | 5-F | Me | Cl | 26.2 | 31.5 | 60.5 | 30.9 | 37.8 | 61.0 |
| 9b | 5-Cl | Me | Cl | 31.7 | 30.4 | 34.4 | 32.6 | 35.2 | 42.8 |
| 9c | 5-Br | Me | Cl | 18.7 | 1.9 | 40.9 | 6.8 | 53.4 | 35.2 |
| 9d | 6-F | Me | Cl | 15.1 | 0.0 | 26.9 | 14.8 | 37.5 | 55.8 |
| 9e | 6-F | Et | Cl | 13.2 | 14.3 | 10.8 | 11.2 | 10.0 | 29.2 |
| 9f | 6-Cl | Me | Cl | 24.6 | 26.7 | 31.5 | 33.1 | 37.5 | 68.1 |
| 9g | 6-Cl | Et | Cl | 12.4 | 12.2 | 10.5 | 6.5 | 7.5 | 61.4 |
| 9h | 6-Br | Et | Cl | 17.0 | 12.5 | 1.8 | 11.8 | 13.2 | 21.6 |
| 9i | 5-Me | Et | Cl | 21.9 | 11.7 | 35.2 | 35.7 | 29.1 | 56.8 |
| 10a | 4-Me | Me | Br | 30.6 | 29.6 | 36.7 | 29.8 | 31.6 | 58.9 |
| 10b | 5-F | Me | Br | 22.2 | 22.8 | 33.4 | 26.2 | 26.4 | 45.8 |
| 10c | 5-Cl | Me | Br | 10.6 | 7.0 | 16.8 | 0.0 | 29.4 | 45.8 |
| 10d | 5-Br | Me | Br | 63.5 | 33.1 | 87.6 | 71.9 | 72.2 | 74.2 |
| 10e | 6-F | Me | Br | 23.8 | 14.0 | 20.5 | 27.9 | 22.1 | 58.2 |
| 10f | 6-F | Et | Br | 35.9 | 28.6 | 25.9 | 30.7 | 31.7 | 64.1 |
| 10g | 6-Cl | Me | Br | 30.7 | 25.0 | 32.8 | 33.6 | 40.3 | 71.4 |
| 10h | 6-Cl | Et | Br | 25.1 | 28.8 | 18.2 | 25.3 | 22.5 | 63.7 |
| 10i | 6-Br | Et | Br | 27.6 | 21.9 | 1.75 | 24.2 | 22.3 | 48.4 |
| Osthole | / | / | / | 31.3 | 61.2 | 70.4 | 92.3 | 57.0 | 66.5 |
| Boscalid | / | / | / | 92.8 | 57.6 | 99.9 | 25.5 | 40.9 | 87.3 |
| Carbendazim | / | / | / | 6.4 | 59.6 | 99.9 | 99.9 | 99.9 | 99.9 |
| Pathogen | Compound | Toxic Regression | R | EC50 (μg/mL) | 95% Confidence Interval |
|---|---|---|---|---|---|
| Alternaria leaf spot | 4a | Y = 2.7969 + 1.7139X | 0.9802 | 19.2928 | 10.2574~36.2873 |
| 5a | Y = 3.9921 + 1.8926X | 0.9974 | 3.4086 | 3.1301~3.7119 | |
| 8d | Y = 1.9984 + 2.0046X | 0.9904 | 31.4339 | 24.7935~39.8527 | |
| Boscalid | Y = 5.1084 + 1.0376X | 0.9935 | 0.7862 | 0.6462~0.9566 | |
| Carbendazim | Y = -1.8843 + 5.7567X | 0.9265 | 15.6994 | 5.6810~43.3849 | |
| Alternaria solani | 5a | Y = 3.8271 + 1.0526X | 0.9977 | 13.0099 | 11.8507~14.2825 |
| Boscalid | Y = 4.3437 + 0.4903X | 0.9806 | 21.8016 | 12.7424~37.3012 | |
| Carbendazim | Y = 3.2290 + 2.5855X | 0.9688 | 4.8412 | 3.3993~6.8947 | |
| Botrytis cinerea | 4a | Y = 5.5662 + 1.2805X | 0.9413 | 0.3613 | 0.0753~1.7329 |
| 5a | Y = 4.6370 + 6.9223X | 0.9167 | 1.1283 | 0.4899~2.5988 | |
| 8d | Y = 0.7267 + 3.0369X | 0.9837 | 25.5341 | 19.8748~32.8049 | |
| Boscalid | Y = 5.2263 + 0.7489X | 0.9810 | 0.4986 | 0.3268~0.7608 | |
| Azoxystrobin | Y = 4.4507 + 0.8502X | 0.9921 | 4.3516 | 3.4330~5.5160 | |
| Colletotrichum lagenarium | 4a | Y = 4.1129 + 1.5735 X | 0.9967 | 3.6625 | 3.2373~4.1435 |
| 5a | Y = 3.6409 + 1.494X | 0.9232 | 8.1215 | 4.8507~13.5978 | |
| 8c | Y = 4.8309 + 1.5299X | 0.9954 | 1.2899 | 1.0572~1.5739 | |
| 8d | Y = 2.8392 + 2.2448X | 0.9997 | 9.1740 | 8.8047~9.5588 | |
| Azoxystrobin | Y = 4.2298 + 0.4299X | 0.9968 | 61.8611 | 49.2272~77.7376 | |
| Boscalid | Y = 2.9242 + 1.351X | 0.9673 | 34.3930 | 18.9576~62.3960 | |
| Gibberella zeae | 5a | Y = 5.1780 + 1.0649X | 0.9090 | 0.6805 | 0.2148~2.1553 |
| Carbendazim | Y = 6.1001 + 8.7644X | 0.9653 | 0.7490 | 0.4996~1.1228 | |
| Rhizoctonia solani | 5a | Y = 5.1533 + 0.7519X | 0.9603 | 0.6215 | 0.1817~2.1250 |
| Boscalid | Y = 5.1182 + 0.5510X | 0.9942 | 0.6103 | 0.4943~0.7535 | |
| Carbendazim | Y = 5.2412 + 4.4774X | 0.9993 | 0.8833 | 0.8410~0.9279 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-R.; Liu, J.-M.; Gao, Y.; Shi, Z.; Nie, K.-R.; Guo, D.; Deng, F.; Zhang, H.-F.; Ali, A.S.; Zhang, M.-Z.; et al. Discovery of Novel Pimprinine and Streptochlorin Derivatives as Potential Antifungal Agents. Mar. Drugs 2022, 20, 740. https://doi.org/10.3390/md20120740
Liu J-R, Liu J-M, Gao Y, Shi Z, Nie K-R, Guo D, Deng F, Zhang H-F, Ali AS, Zhang M-Z, et al. Discovery of Novel Pimprinine and Streptochlorin Derivatives as Potential Antifungal Agents. Marine Drugs. 2022; 20(12):740. https://doi.org/10.3390/md20120740
Chicago/Turabian StyleLiu, Jing-Rui, Jia-Mu Liu, Ya Gao, Zhan Shi, Ke-Rui Nie, Dale Guo, Fang Deng, Hai-Feng Zhang, Abdallah S. Ali, Ming-Zhi Zhang, and et al. 2022. "Discovery of Novel Pimprinine and Streptochlorin Derivatives as Potential Antifungal Agents" Marine Drugs 20, no. 12: 740. https://doi.org/10.3390/md20120740