Molecular Network Guided Cataloging of the Secondary Metabolome of Selected Egyptian Red Sea Soft Corals
Abstract
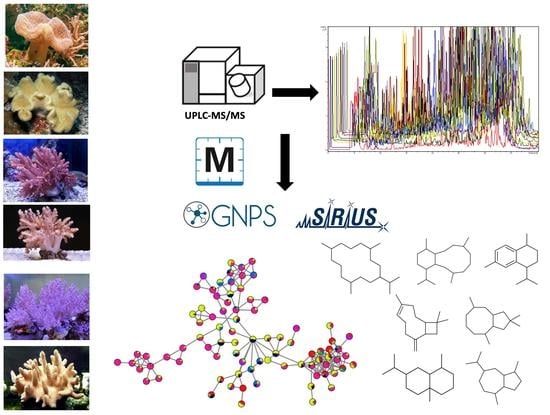
:1. Introduction
2. Results and Discussion
2.1. Cembrane Diterpenes
2.2. Eunicellin Diterpenes
2.3. Sesquiterpenes
2.4. Sterols
2.5. Others
3. Materials and Methods
3.1. Soft Coral Material
3.2. Chemicals and Reagents
3.3. Soft Corals Extraction and Sample Preparation for UPLC-MS Analysis
3.4. UPLC–HRMS/MS Analysis
3.5. Feature-Based Molecular Networking and Compounds Dereplication
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DiBattista, J.D.; Roberts, M.B.; Bouwmeester, J.; Bowen, B.W.; Coker, D.J.; Lozano-Cortés, D.F.; Howard Choat, J.; Gaither, M.R.; Hobbs, J.P.A.; Khalil, M.T. A review of contemporary patterns of endemism for shallow water reef fauna in the Red Sea. J. Biogeogr. 2016, 43, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Porzel, A.; Al-Hammady, M.A.; Hegazy, M.-E.F.; Meyer, A.; Mohamed, T.A.; Westphal, H.; Wessjohann, L.A. Soft corals biodiversity in the Egyptian Red Sea: A comparative MS and NMR metabolomics approach of wild and aquarium grown species. J. Proteome Res. 2016, 15, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Scesa, P.D.; Lin, Z.; Schmidt, E.W. Ancient defensive terpene biosynthetic gene clusters in the soft corals. Nat. Chem. Biol. 2022, 18, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Mohamed, T.A.; Alhammady, M.A.; Shaheen, A.M.; Reda, E.H.; Elshamy, A.I.; Aziz, M.; Paré, P.W. Molecular architecture and biomedical leads of terpenes from red sea marine invertebrates. Mar. Drugs 2015, 13, 3154–3181. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.A.; Elshamy, A.I.; Abdel-Tawab, A.M.; AbdelMohsen, M.M.; Ohta, S.; Pare, P.W.; Hegazy, M.-E.F. Oxygenated Cembrene Diterpenes from Sarcophyton convolutum: Cytotoxic Sarcoconvolutum AE. Mar. Drugs 2021, 19, 519. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.F.; Eldeen, A.M.G.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Paré, P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegazy, M.-E.F.; Elshamy, A.I.; Mohamed, T.A.; Hamed, A.R.; Ibrahim, M.A.; Ohta, S.; Paré, P.W. Cembrene diterpenoids with ether linkages from Sarcophyton ehrenbergi: An anti-proliferation and molecular-docking assessment. Mar. Drugs 2017, 15, 192. [Google Scholar] [CrossRef]
- Gross, H.; König, G.M. Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochem. Rev. 2006, 5, 115–141. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Mohamed, T.A.; Elshamy, A.I.; Hamed, A.R.; Ibrahim, M.A.; Ohta, S.; Umeyama, A.; Paré, P.W.; Efferth, T. Sarcoehrenbergilides D–F: Cytotoxic cembrene diterpenoids from the soft coral Sarcophyton ehrenbergi. RSC Adv. 2019, 9, 27183–27189. [Google Scholar] [CrossRef] [Green Version]
- Chill, L.; Berrer, N.; Benayahu, Y.; Kashman, Y. Eunicellin diterpenes from two Kenyan soft corals. J. Nat. Prod. 2005, 68, 19–25. [Google Scholar] [CrossRef]
- Hassan, H.M.; Khanfar, M.A.; Elnagar, A.Y.; Mohammed, R.; Shaala, L.A.; Youssef, D.T.; Hifnawy, M.S.; El Sayed, K.A. Pachycladins A−E, prostate cancer invasion and migration inhibitory eunicellin-based diterpenoids from the Red Sea soft coral Cladiella pachyclados. J. Nat. Prod. 2010, 73, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.A.; Cooke, I.; Wilson, D.T.; Miller, D.J.; Peigneur, S.; Tytgat, J.; Field, M.; Takjoo, R.; Smout, M.J.; Loukas, A. Newly Discovered Peptides from the Coral Heliofungia actiniformis Show Structural and Functional Diversity. J. Nat. Prod. 2022, 85, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Putra, M.Y.; Ianaro, A.; Panza, E.; Bavestrello, G.; Cerrano, C.; Fattorusso, E.; Taglialatela-Scafati, O. Sinularioside, a triacetylated glycolipid from the Indonesian soft coral Sinularia sp., is an inhibitor of NO release. Bioorg. Med. Chem. Lett. 2012, 22, 2723–2725. [Google Scholar] [CrossRef]
- Gao, C.; Yi, X.; Huang, R.; Yan, F.; He, B.; Chen, B. Alkaloids from corals. Chem. Biodivers. 2013, 10, 1435–1447. [Google Scholar] [CrossRef]
- Burkhardt, I.; de Rond, T.; Chen, P.Y.-T.; Moore, B.S. Ancient plant-like terpene biosynthesis in corals. Nat. Chem. Biol. 2022, 18, 664–669. [Google Scholar] [CrossRef]
- Ali, S.E.; Farag, M.A. Expanding Metabolomics Applications to Address Issues in Marine Ecology and Natural Products Chemistry. In Encyclopedia of Marine Biotechnology; Kim, S.-K., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; Volume 3, pp. 1827–1842. [Google Scholar]
- Ramos, A.E.F.; Evanno, L.; Poupon, E.; Champy, P.; Beniddir, M.A. Natural products targeting strategies involving molecular networking: Different manners, one goal. RSC Adv. 2019, 36, 960–980. [Google Scholar]
- Farag, M.A.; Meyer, A.; Ali, S.E.; Salem, M.A.; Giavalisco, P.; Westphal, H.; Wessjohann, L.A. Comparative metabolomics approach detects stress-specific responses during coral bleaching in soft corals. J. Proteome Res. 2018, 17, 2060–2071. [Google Scholar] [CrossRef]
- Farag, M.A.; Maamoun, A.A.; Meyer, A.; Wessjohann, L.A. Salicylic acid and its derivatives elicit the production of diterpenes and sterols in corals and their algal symbionts: A metabolomics approach to elicitor SAR. Metabolomics 2018, 14, 127. [Google Scholar] [CrossRef]
- Maloney, K.N.; Botts, R.T.; Davis, T.S.; Okada, B.K.; Maloney, E.M.; Leber, C.A.; Alvarado, O.; Brayton, C.; Caraballo-Rodríguez, A.M.; Chari, J.V. Cryptic species account for the seemingly idiosyncratic secondary metabolism of Sarcophyton glaucum specimens collected in Palau. J. Nat. Prod. 2020, 83, 693–705. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Nuzillard, J.-M.; Van Der Hooft, J.J.; Renault, J.-H.; Bertrand, S. Accelerating metabolite identification in natural product research: Toward an ideal combination of liquid chromatography–high-resolution tandem mass spectrometry and NMR profiling, in silico databases, and chemometrics. Anal. Chem. 2018, 91, 704–742. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Böcker, S.; Dührkop, K. Fragmentation trees reloaded. J. Cheminform. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokalahy, E.E.; El-Seedi, H.R.; Farag, M.A. Soft Coral Biodiversity in the Red Sea Family Alcyoniidae: A Biopharmaceutical and Ecological Perspective. In Biodiversity and Chemotaxonomy; Ramawat, K.G., Ed.; Springer: Cham, Switzerland, 2019; pp. 55–85. [Google Scholar]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, Sinularia, and Lobophytum since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.-E.F.; El-Beih, A.A.; Moustafa, A.Y.; Hamdy, A.A.; Alhammady, M.A.; Selim, R.M.; Abdel-Rehim, M.; Paré, P.W. Cytotoxic cembranoids from the Red Sea soft coral Sarcophyton glaucum. Nat. Prod. Commun. 2011, 6, 1809–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morlock, G.E.; Ziltener, A.; Geyer, S.; Tersteegen, J.; Mehl, A.; Schreiner, T.; Kamel, T.; Brümmer, F. Evidence that Indo-Pacific bottlenose dolphins self-medicate with invertebrates in coral reefs. iScience 2022, 25, 104271. [Google Scholar] [CrossRef]
- Elkhateeb, A.; El-Beih, A.A.; Gamal-Eldeen, A.M.; Alhammady, M.A.; Ohta, S.; Paré, P.W.; Hegazy, M.-E.F. New terpenes from the Egyptian soft coral Sarcophyton ehrenbergi. Mar. Drugs 2014, 12, 1977–1986. [Google Scholar] [CrossRef]
- Cheng, Y.-B.; Shen, Y.-C.; Kuo, Y.-H.; Khalil, A.T. Cembrane diterpenoids from the Taiwanese soft coral Sarcophyton stolidotum. J. Nat. Prod. 2008, 71, 1141–1145. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chuang, C.-T.; Wen, Z.-H.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Duh, C.-Y. Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 2010, 18, 3379–3386. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chen, A.-N.; Shao, C.-L.; Li, L.; Xu, Y.; Quian, P.-Y. Chemical constituents of soft coral Sarcophyton infundibuliforme from the South China Sea. Biochem. Syst. Ecol. 2011, 39, 853–856. [Google Scholar] [CrossRef]
- Yin, S.W.; Shi, Y.P.; Li, X.M.; Wang, B.G. A New Cembranoid Diterpene and Other Related Metabolites from the South-China-Sea Soft Coral Lobophytum crassum. Helv. Chim. Acta 2006, 89, 567–572. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 649–652. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Huang, I.-C.; Chiang, M.Y.-N.; Hwang, T.-L.; Kung, T.-H.; Lin, C.-S.; Sheu, J.-H.; Sung, P.-J. Briaviodiol A, a new Cembranoid from a softcoral Briareum violacea. Chem. Pharm. Bull. 2010, 58, 1666–1668. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-Y.; Chen, P.-W.; Chen, H.-P.; Wang, S.-K.; Duh, C.-Y. New cembranolides from the Dongsha Atoll soft coral Lobophytum durum. Mar. Drugs 2011, 9, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, A.-H.; Huang, H.; Li, J.; Mollo, E.; Gavagnin, M.; Cimino, G.; Gu, Y.-C.; Guo, Y.-W. Diterpenoids from the Hainan Soft Coral Sinularia parva. Helv. Chim. Acta 2009, 92, 1341–1348. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.; Zhao, F.; Cheng, S.; Xiang, Z.; Dong, J.; Huang, K.; Yan, P. Four New 7,8-epoxycembranoids from a Chinese soft coral Lobophytum sp. Chem. Pharm. Bull. 2013, 61, 1323–1328. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.-F.; Chen, M.-J.; Liang, D.-E.; Shi, L.-M.; Ying, Y.-M.; Shan, W.-G.; Li, G.-Q.; Zhan, Z.-J. Streptomyces albogriseolus SY67903 produces eunicellin diterpenoids structurally similar to terpenes of the gorgonian Muricella sibogae, the bacterial source. J. Nat. Prod. 2020, 83, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Ru, T.; Cai, Y.-S.; Li, H.; Tang, W.; Wang, H.; Guo, Y.-W. Further new eunicellin-based diterpenoids from the Guangxi Weizhou soft coral Cladiella krempfi. Fitoterapia 2018, 131, 200–203. [Google Scholar] [CrossRef]
- Alam, M.; Sharma, P.; Zektzer, A.S.; Martin, G.E.; Ji, X.; van der Helm, D. Sclerophytin C-F: Isolation and Structures of Four New Diterpenes from the Soft Coral Sclerophytum capitalis. J. Org. Chem. 1989, 54, 1896–1900. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Chen, W.-F.; Wen, Z.-H.; Hwang, T.-L.; Zhang, Z.-J.; Sung, P.-J. New bioactive Δ11(17)-furanoeunicellins from an octocoral Cladiella sp. Phytochem. Lett. 2019, 33, 31–35. [Google Scholar] [CrossRef]
- Rao, C.B.; Rao, D.S.; Satyanarayana, C.; Rao, D.V.; Kassühlke, K.E.; Faulkner, D.J. New cladiellane diterpenes from the soft coral Cladiella australis of the Andaman and Nicobar Islands. J. Nat. Prod. 1994, 57, 574–580. [Google Scholar] [CrossRef]
- Lai, D.; Liu, D.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Antifouling Eunicellin-Type Diterpenoids fom the Gorgonian Astrogorgia sp. J. Nat. Prod. 2012, 75, 1595–1602. [Google Scholar] [CrossRef]
- Cai, Y.-S.; Yao, L.-G.; Di Pascale, A.; Irace, C.; Mollo, E.; Taglialatela-Scafati, O.; Guo, Y.-W. Polyoxygenated diterpenoids of the eunicellin-type from the Chinese soft coral Cladiella krempfi. Tetrahedron 2013, 69, 2214–2219. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, W.-F.; Wen, Z.-H.; Lu, M.-C.; Wang, W.-H.; Li, J.-J.; Wu, Y.-C.; Sung, P.-J. Cladieunicellins M–Q, new eunicellins from Cladiella sp. Mar. Drugs 2014, 12, 2144–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.-W.; Wu, Y.-C.; Chiang, M.Y.; Su, J.-H.; Wang, W.-H.; Fan, T.-Y.; Sheu, J.-H. Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Tetrahedron 2009, 65, 7016–7022. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kusatsu, T.; Tsuha, K.; Hamada, T.; Okamura, H.; Furukawa, T.; Akiyama, S.-I.; Doe, M.; Morimoto, Y.; Iwase, F.; et al. Cytotoxic eunicellin-type diterpenes from the soft coral Litophyton viscudium. Heterocycles 2011, 83, 2149–2155. [Google Scholar] [CrossRef]
- Shih, F.-Y.; Chen, T.-H.; Lu, M.-C.; Chen, W.-F.; Wen, Z.-H.; Kuo, Y.-H.; Sung, P.-J. Cladieunicellins K and L, new eunicellin-based diterpenoids from an octocoral Cladiella sp. Int. J. Mol. Sci. 2013, 14, 21781–21789. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Yamada, K.; Futatsugi, K.; Kotsuki, H.; Shibata, K. Litophynins F, G, and H, Three New Diterpenoids from a Soft Coral Litophyton sp. Heterocycles 1991, 32, 29–32. [Google Scholar] [CrossRef]
- Ospina, C.A.; Rodríguez, A.D.; Ortega-Barria, E.; Capson, T.L. Briarellins J−P and Polyanthellin A: New Eunicellin-Based Diterpenes from the Gorgonian Coral Briareum polyanthes and Their Antimalarial Activity. J. Nat. Prod. 2003, 66, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Su, J.-H.; Huang, C.-Y.; Tai, C.-J.; Sung, P.-J.; Liaw, C.-C.; Sheu, J.-H. Simplexins P–S, eunicellin-based diterpenes from the soft coral Klyxum simplex. Mar. Drugs 2012, 10, 1203–1211. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.E.; Amlani, A.; Dewi, A.S.; Patrick, B.O.; van Ofwegen, L.; Mui, A.L.-F.; Andersen, R.J. Australin E Isolated from the Soft Coral Cladiella sp. Collected in Pohnpei Activates the Inositol 5-Phosphatase SHIP1. Aust. J. Chem. 2010, 63, 895–900. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chang, S.-M.; Huang, C.-Y.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. Hirsutalins A− H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010, 73, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Chen, B.-W.; Huang, C.-Y.; Dai, C.-F.; Hwang, T.-L.; Sheu, J.-H. Eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2013, 76, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Le Bideau, F.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Leng, X.; Ouyang, H. Chemical diversity and biological activity of secondary metabolites from soft coral genus Sinularia since 2013. Mar. Drugs 2021, 19, 335. [Google Scholar] [CrossRef]
- Wang, G.-H.; Ahmed, A.F.; Sheu, J.-H.; Duh, C.-Y.; Shen, Y.-C.; Wang, L.-T. Suberosols A− D, four new sesquiterpenes with β-caryophyllene skeletons from a Taiwanese gorgonian coral Subergorgia suberosa. J. Nat. Prod. 2002, 65, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Huang, Y.-C.; Wen, Z.-H.; Chiou, S.-F.; Wang, S.-K.; Hsu, C.-H.; Dai, C.-F.; Duh, C.-Y. Novel sesquiterpenes and norergosterol from the soft corals Nephthea erecta and Nephthea chabroli. Tetrahedron Lett. 2009, 50, 802–806. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Asteriscane-type sesquiterpenoids from the soft coral Sinularia capillosa. J. Nat. Prod. 2013, 76, 1753–1763. [Google Scholar] [CrossRef]
- Anjaneyulu, A.; Chaturvedula, V.S.P. New Sesqui-and Diterpenoids from the Soft Coral Nephthea chabroli of Indian Coast. Indian J. Chem. Sect. B 2013, 34B, 32. [Google Scholar]
- Zheng, J.-J.; Shao, C.-L.; Chen, M.; Gan, L.-S.; Fang, Y.-C.; Wang, X.-H.; Wang, C.-Y. Ochracenoids A and B, guaiazulene-based analogues from gorgonian Anthogorgia ochracea collected from the South China Sea. Mar. Drugs 2014, 12, 1569–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coll, J.C.; Bowden, B.F.; Tapiolas, D.M.; Willis, R.H.; Djura, P.; Streamer, M.; Trott, L. Studies of Australian soft corals—XXXV: The terpenoid chemistry of soft corals and its implications. Tetrahedron 1985, 41, 1085–1092. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wang, S.-K.; Wen, Z.-H.; Dai, C.-F.; Duh, C.-Y. Three new eudesmanoids from the Formosan soft coral Nephthea erecta. J. Asian Nat. Prod. Res. 2009, 11, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Sarma, N.S.; Krishna, M.S.; Pasha, S.G.; Rao, T.S.P.; Venkateswarlu, Y.; Parameswaran, P. Marine metabolites: The sterols of soft coral. Chem. Rev. 2009, 109, 2803–2828. [Google Scholar] [CrossRef]
- Cardoso-Martínez, F.; José, M.; Díaz-Marrero, A.R.; Darias, J.; D’Croz, L.; Jiménez-Antón, M.D.; Corral, M.J.; García, R.; Alunda, J.M.; Cueto, M. Oxysterols from an octocoral of the genus Gorgonia from the eastern Pacific of Panama. RSC Adv. 2016, 6, 38579–38591. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ibrahim, A.; Arafa, A.S. Anti-H5N1 virus metabolites from the Red Sea soft coral, Sinularia candidula. Tetrahedron Lett. 2013, 54, 2377–2381. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Chu, M.-J.; Sheu, J.-H. Cytotoxic sterols from the soft coral Nephthea erecta. J. Nat. Prod. 1998, 61, 1022–1024. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Huong, P.T.M.; Van Thanh, N.; Chi, N.T.P.; Dang, N.H.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; van Kiem, P.; van Minh, C. Cytotoxic steroids from the Vietnamese soft coral Sinularia conferta. Chem. Pharm. Bull. 2017, 65, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-X.; Yan, S.-J.; Zhang, G.-W.; Su, J.-Y.; Zeng, L.-M. Isolation of new polyhydroxylated sterol from soft coral Sarcophyton crassocaule Mosre. Chem. J. Chin. Univ. 2007, 28, 686–688. [Google Scholar]
- Cheng, S.-Y.; Dai, C.-F.; Duh, C.-Y. New 4-methylated and 19-oxygenated steroids from the Formosan soft coral Nephthea erecta. Steroids 2007, 72, 653–659. [Google Scholar] [CrossRef]
- Songzhi, D.; Chunlei, T.; Dingjun, X. Studies on the chemical constituents of the sponge Biemna fortis from the South China Sea. Chin. J. Mar. Drugs 1999, 18, 4–6. [Google Scholar]
- Zhang, W.; Liu, W.K.; Che, C.-T. Polyhydroxylated steroids and other constituents of the soft coral Nephthea chabroli. Chem. Pharm. Bull. 2003, 51, 1009–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, J.; Sabdono, A.; Imhoff, J.F. Corals as source of bacteria with antimicrobial activity. J. Coast. Dev. 2008, 11, 121–130. [Google Scholar]
- Li, J.; Dong, J.-D.; Yang, J.; Luo, X.-M.; Zhang, S. Detection of polyketide synthase and nonribosomal peptide synthetase biosynthetic genes from antimicrobial coral-associated actinomycetes. Antonie Van Leeuwenhoek 2014, 106, 623–635. [Google Scholar] [CrossRef] [PubMed]
- McClintock, J.B.; Baker, B.J. Marine Chemical Ecology, 1st ed.; CRC Press: Boca Raton, USA, 2001. [Google Scholar]
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef]
- Macfadyen, L. Alcyonaria (Stolonifera, Alcyonacea, Telestacea and Gorgonacea). 1935. Available online: https://biostor.org/reference/175067 (accessed on 1 August 2022).
- Thomson, J.A.; Dean, L.M. The Alcyonacea of the Siboga-Expedition: With an Addendum to the Gorgonacea. In Siboga Expedition, 1899–1900; E.J. Brill: Leiden, The Netherlands, 1931. [Google Scholar]
- Gohar, H.A.F. Studies on the Xeniidae of the Red Sea; Publications of the Marine Biological Station: Ghardaqa (Red Sea), Egypt, 1940. [Google Scholar]
- Reinicke, G.B. Xeniidae des Roten Meeres (Octocorallia, Alcyonacea): Beiträge zur Systematik und Ökologie; Westarp Wissenschaften: Hohenwarsleben, Germany, 1995. [Google Scholar]
- Verseveldt, J. A Revision of the Genus Sarcophyton Lesson (Octocorallia, Alcyonacea); Brill: Leiden, The Netherlands, 1982. [Google Scholar]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea; New Litho: Melbourne, Australia, 2001. [Google Scholar]
- Benayahu, Y.; Loya, Y.J.T.B.B. Sexual reproduction of a soft coral: Synchronous and brief annual spawning of Sarcophyton glaucum (Quoy & Gaimard, 1833). Biol. Bull. 1986, 170, 32–42. [Google Scholar]
- Hegazi, N.M.; Radwan, R.A.; Ali, S.M.; Saad, H.H. Molecular networking aided metabolomic profiling of beet leaves using three extraction solvents and in relation to its anti-obesity effects. J. Adv. Res. 2020, 24, 545–555. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Saad, H.H.; Marzouk, M.M.; Abdel Rahman, M.F.; El Bishbishy, M.H.; Zayed, A.; Ulber, R.; Ezzat, S.M. Molecular networking leveraging the secondary metabolomes space of Halophila stipulaceae (Forsk.) Aschers. and Thalassia hemprichii (Ehrenb. ex Solms) Asch. in tandem with their chemosystematics and antidiabetic potentials. Mar. Drugs 2021, 19, 279. [Google Scholar] [CrossRef]
- Garg, N.; Kapono, C.A.; Lim, Y.W.; Koyama, N.; Vermeij, M.J.; Conrad, D.; Rohwer, F.; Dorrestein, P.C. Mass spectral similarity for untargeted metabolomics data analysis of complex mixtures. Int. J. Mass Spectrom. 2015, 377, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using CSI: FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef] [Green Version]






| Soft coral Species | Sample Code | Extraction Yield (g) | Location | Coordinates | |
|---|---|---|---|---|---|
| Latitude | Longitude | ||||
| Sinularia brassica | SB | 2.8 | Northern Safaga | 33°56′33.88″ E | 26°44′11.92″ N |
| Sinularia gardeneri | SIG | 3.1 | Northern Safaga | 33°56′33.88″ E | 26°44′11.92″ N |
| Sinularia leptoclados | SIL | 3.4 | NIOF-Hurghada | 33°46′25.30″ E | 27°17′12.07″ N |
| Sarcophyton ehrenbergi | SE | 3.8 | NIOF-Hurghada | 33°46′25.30″ E | 27°17′12.07″ N |
| Sarcophyton roseum | SAR | 3.7 | NIOF-Hurghada | 33°46′25.30″ E | 27°17′12.07″ N |
| Sarcophyton acutum | SAA | 3.5 | NIOF-Hurghada | 33°46′25.30″ E | 27°17′12.07” N |
| Litophyton mollis | LM | 3.2 | Northern Safaga | 33°56′33.88″ E | 26°44′11.92″ N |
| Cladiella pachyclados | CLP | 2.5/2.4 | Marsa Alam | 34°53′50.03″ E | 25°4′36.26″ N |
| Lobophytum pauciflorum | LOPH | 2.2 | Marsa Alam | 34°53′50.03″ E | 25°4′36.26″ N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegazi, N.M.; Mohamed, T.A.; Saad, H.H.; Al-Hammady, M.A.; Hussien, T.A.; Hegazy, M.-E.F.; Gross, H. Molecular Network Guided Cataloging of the Secondary Metabolome of Selected Egyptian Red Sea Soft Corals. Mar. Drugs 2022, 20, 630. https://doi.org/10.3390/md20100630
Hegazi NM, Mohamed TA, Saad HH, Al-Hammady MA, Hussien TA, Hegazy M-EF, Gross H. Molecular Network Guided Cataloging of the Secondary Metabolome of Selected Egyptian Red Sea Soft Corals. Marine Drugs. 2022; 20(10):630. https://doi.org/10.3390/md20100630
Chicago/Turabian StyleHegazi, Nesrine M., Tarik A. Mohamed, Hamada H. Saad, Montaser A. Al-Hammady, Taha A. Hussien, Mohamed-Elamir F. Hegazy, and Harald Gross. 2022. "Molecular Network Guided Cataloging of the Secondary Metabolome of Selected Egyptian Red Sea Soft Corals" Marine Drugs 20, no. 10: 630. https://doi.org/10.3390/md20100630