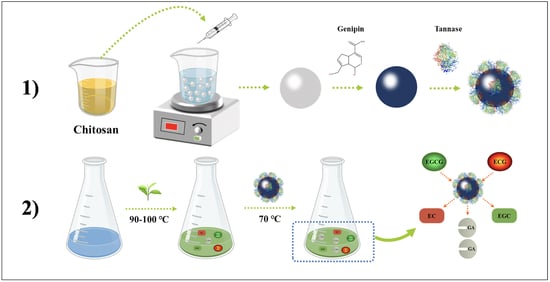
Chitosan Activated with Genipin: A Nontoxic Natural Carrier for Tannase Immobilization and Its Application in Enhancing Biological Activities of Tea Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of the Working Activation Conditions for Chitosan Beads
2.2. FTIR Analysis
2.3. TGA Analysis
2.4. Determination of the Working Conditions for Tannase Immobilization
2.5. Enzymatic Properties of Tannase and GP–CS–Tannase
2.6. Reusability and Storage Stability of GP–CS–Tannase
2.7. Effect of Extraction Temperature and Time on Catechins in Green Tea
2.8. Effect of Tannase Treatment on the Biological Activity of Tea Extracts
2.8.1. Effect of Enzymatic Treatment on the Total Phenols of Tea Extracts
2.8.2. Effect of Enzymatic Treatment on the Reducing Capacities of Tea Extracts
2.8.3. Hydroxyl Radical-Scavenging Effect of Tea Extract
2.8.4. Antioxidant Activity by DPPH Radical Scavenging Assay
2.8.5. Inhibitory Effect on α-Amylase and α-Glucosidase Activities
3. Materials and Methods
3.1. Materials and Strain
3.2. Preparation and Activation of Chitosan Beads
3.3. Enzymatic Immobilization
3.4. Activity Assay
3.5. Characterization of CS, GP–CS, and GP–CS–Tannase by FT-IR and TGA
3.6. Assessment of Enzymatic Characteristics, Reusability, and Storage Stability
3.7. Treatment of Tea Extracts with GP–CS–Tannase
3.8. Catechin Composition and Total Polyphenol Content Analysis of Tea Extracts
3.9. Biological Activity Detection of Green Tea Extract
3.9.1. Determination of Reducing Power
3.9.2. Determination of Hydroxyl Radical-Scavenging Capacity
3.9.3. Determination of DPPH Radical-Scavenging Activity
3.9.4. Inhibition Effect on α-Amylase
3.9.5. Inhibition Effect on α-Glucosidase
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jacqueline, S. Oakes, Investigation of Iron Reduction by Green Tea Polyphenols for Application in Soil Remediation. Master’s Theses, University of Connecticut, Storrs, CT, USA, 19 December 2013. [Google Scholar]
- Wu, S.C.; Wang, C.-W.; Hsu, L.-H.; Liang, C. Assessment of green tea reductive degradation of halogenated solvents. Chemosphere 2021, 267, 129196. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Hynes, M.J. The kinetics and mechanisms of the complex formation and antioxidant behaviour of the polyphenols EGCg and ECG with iron(III). J. Inorg. Biochem. 2007, 101, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, Q.; Huang, Y.; Ni, H.; Wu, C.; Xiao, A. Tannase application in secondary enzymatic processing of inferior Tieguanyin oolong tea. Electron. J. Biotechnol. 2017, 28, 87–94. [Google Scholar] [CrossRef]
- Rutter, P.; Stainsby, G. The solubility of tea cream. J. Sci. Food Agric. 1975, 26, 455–463. [Google Scholar] [CrossRef]
- Farag, A.M.; Hassan, S.W.; El-Says, A.M.; Ghanem, K.M. Purification, Characterization and Application of Tannase Enzyme Isolated from Marine Aspergillus nomius GWA5. J. Pure Appl. Microbiol. 2018, 12, 1939–1949. [Google Scholar] [CrossRef] [Green Version]
- Koseki, T.; Otsuka, M.; Mizuno, T.; Shiono, Y. Mutational analysis of Kex2 recognition sites and a disulfide bond in tannase from Aspergillus oryzae. Biochem. Biophys. Res. Commun. 2017, 482, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Bhoite, R.N.; Murthy, P.S. Biodegradation of coffee pulp tannin by Penicillium verrucosum for production of tannase, statistical optimization and its application. Food Bioprod. Process. 2015, 94, 727–735. [Google Scholar] [CrossRef]
- Gayen, S.; Ghosh, U. Purification and characterization of tannin acyl hydrolase produced by mixed solid state fermentation of wheat bran and marigold flower by Penicillium notatum NCIM 923. Biomed. Res. Int. 2013, 2013, 596380. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.E.; Fathy, S.A.; Rashad, M.M.; Ezz, M.K.; Mohammed, A.T. Purification and characterization of a novel tannase produced by Kluyveromyces marxianus using olive pomace as solid support, and its promising role in gallic acid production. Int. J. Biol. Macromol. 2018, 107, 2342–2350. [Google Scholar] [CrossRef]
- Chávez-González, M.; Rodríguez-Durán, L.V.; Balagurusamy, N.; Prado-Barragán, A.; Rodríguez, R.; Contreras, J.C.; Aguilar, C.N. Biotechnological Advances and Challenges of Tannase: An Overview. Food Bioprocess Technol. 2011, 5, 445–459. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Yin, J.-F.; Chen, J.-X.; Wang, F.; Du, Q.-Z.; Jiang, Y.-W.; Xu, Y.-Q. Improving the sweet aftertaste of green tea infusion with tannase. Food Chem. 2016, 192, 470–476. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, Y.-H.; Zhang, F.; Yang, Q.-M.; Weng, H.-F.; Xiao, Q.; Xiao, A.-F. Thermostable Tannase from Aspergillus Niger and Its Application in the Enzymatic Extraction of Green Tea. Molecules 2020, 25, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcı́a-Conesa, M.-T.; Østergaard, P.; Kauppinen, S.; Williamson, G. Hydrolysis of diethyl diferulates by a tannase from Aspergillus oryzae. Carbohydr. Polym. 2001, 44, 319–324. [Google Scholar] [CrossRef]
- Selwal, M.K.; Yadav, A.; Selwal, K.K.; Aggarwal, N.; Gupta, R.; Gautam, S.K. Tannase Production by Penicillium Atramentosum KM under SSF and its Applications in Wine Clarification and Tea Cream Solubilization. Braz. J. Microbiol. 2011, 42, 374–387. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Silva, D.L.J.; Paola, C.M.; Alberto, C.A.; Fernandes, D.S.M.; Patrizia, P.; Bezerra, D.C.L.; Attilio, C. Immobilization of Aspergillus ficuum tannase in calcium alginate beads and its application in the treatment of boldo (Peumus boldus) tea. Int. J. Biol. Macromol. 2018, 118, 1989–1994. [Google Scholar]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. 2020, 107584. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Chen, K.N.; Zhang, J.N.; Hong, R.; Zhang, X.Y.; Huang, J.H.; Wang, S.Z.; Fang, B.S. Preparation and evaluation of a polymer–metal–enzyme hybrid nanowire forthe immobilization of multiple oxidoreductases. J. Chem. Technol. Biotechnol. 2019, 94, 795–803. [Google Scholar] [CrossRef]
- Wu, C.; Xu, C.; Ni, H.; Yang, Q.; Cai, H.; Xiao, A. Preparation and characterization of tannase immobilized onto carboxyl-functionalized superparamagnetic ferroferric oxide nanoparticles. Bioresour. Technol. 2016, 205, 67–74. [Google Scholar] [CrossRef]
- Jana, A.; Halder, S.K.; Ghosh, K.; Paul, T.; Vágvölgyi, C.; Mondal, K.C.; Das Mohapatra, P.K. Tannase Immobilization by Chitin-Alginate Based Adsorption-Entrapment Technique and Its Exploitation in Fruit Juice Clarification. Food Bioprocess Technol. 2015, 8, 2319–2329. [Google Scholar] [CrossRef]
- Klein, M.P.; Hackenhaar, C.R.; Lorenzoni, A.S.G.; Rodrigues, R.C.; Costa, T.M.H.; Ninow, J.L.; Hertz, P.F. Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and beta-D-galactosidase immobilization. Carbohydr. Polym. 2016, 137, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.N.; Pereira, F.M.; Rocha, M.A.; Ribeiro, J.G.; Diz, F.M.; Monteiro, W.F.; Ligabue, R.A.; Severino, P.; Fricks, A.T. Immobilization and characterization of Horseradish Peroxidase into Chitosan and Chitosan/PEG nanoparticles: A comparative study. Process. Biochem. 2020, 98, 160–171. [Google Scholar] [CrossRef]
- Flores, E.E.E.; Cardoso, F.D.; Siqueira, L.B.; Ricardi, N.C.; Costa, T.H.; Rodrigues, R.C.; Klein, M.P.; Hertz, P.F. Influence of reaction parameters in the polymerization between genipin and chitosan for enzyme immobilization. Process. Biochem. 2019, 84, 73–80. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Jing, Z.-W.; Ma, Z.-W.; Li, C.; Jia, Y.-Y.; Luo, M.; Ma, X.-X.; Zhou, S.-Y.; Zhang, B.-L. Chitosan cross-linked with poly(ethylene glycol)dialdehyde via reductive amination as effective controlled release carriers for oral protein drug delivery. Bioorganic Med. Chem. Lett. 2017, 27, 1003–1006. [Google Scholar] [CrossRef]
- Bor, G.; Mytych, J.; Zebrowski, J.; Wnuk, M.; Şanlı-Mohamed, G. Cytotoxic and cytostatic side effects of chitosan nanoparticles as a non-viral gene carrier. Int. J. Pharm. 2016, 513, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, B.; Singh, H.; Khatri, M.; Singh, G.; Arya, S.K. Immobilization of keratinase on chitosan grafted-beta-cyclodextrin for the improvement of the enzyme properties and application of free keratinase in the textile industry. Int. J. Biol. Macromol. 2020, 165, 1099–1110. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Trizna, E.Y.; Pankova, S.M.; Agafonova, M.N.; Chirkova, M.N.; Vasileva, O.S.; Akhmetov, N.; Shubina, V.V.; Porfiryev, A.G.; et al. Anti-biofilm and wound-healing activity of chitosan-immobilized Ficin. Int. J. Biol. Macromol. 2020, 164, 4205–4217. [Google Scholar] [CrossRef]
- Yang, J.; Xu, P.; Long, L.; Ding, S. Production of lactobionic acid using an immobilized cellobiose dehydrogenase/laccase system on magnetic chitosan spheres. Process. Biochem. 2021, 100, 1–9. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Enhanced mechanical strength of chitosan hydrogel beads by impregnation with carbon nanotubes. Carbon 2009, 47, 2933–2936. [Google Scholar] [CrossRef]
- Wahba, M.I. Porous chitosan beads of superior mechanical properties for the covalent immobilization of enzymes. Int. J. Biol. Macromol. 2017, 105, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.; Oliveira, R.; Coelho, S.; Musso, C.; Soares, A.M.; Domingues, I.; Nogueira, A.J. From sub cellular to community level: Toxicity of glutaraldehyde to several aquatic organisms. Sci. Total. Environ. 2014, 470–471, 147–158. [Google Scholar] [CrossRef]
- Djerassi, C.; Nakano, T.; James, A.N.; Zalkow, L.H.; Shoolery, J.N. Terpenoids. XLVII. The structure of genipin. J. Org. Chem. 1961, 26, 1192–1206. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; García-Parra, E.; Vela-Gutiérrez, G.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications. Catalysts 2019, 19, 1035. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.-W.; Huang, R.-N.; Huang, L.L.; Tsai, C.-C. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Cavello, I.A.; Contreras-Esquivel, J.C.; Cavalitto, S.F. Immobilization of a keratinolytic protease from Purpureocillium lilacinum on genipin activated-chitosan beads. Process. Biochem. 2014, 49, 1332–1336. [Google Scholar] [CrossRef]
- Khan, N.; Maseet, M.; Basir, S.F. Synthesis and characterization of biodiesel from waste cooking oil by lipase immobilized on genipin cross-linked chitosan beads: A green approach. Int. J. Green Energy 2019, 17, 84–93. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process. Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Alamsyah, G.; Albels, V.A.; Sahlan, M.; Hermansyah, H. Effect of chitosan’s amino group in adsorption-crosslinking immobilization of lipase enzyme on resin to catalyze biodiesel synthesis. Energy Procedia 2017, 136, 47–52. [Google Scholar] [CrossRef]
- Olshansky, Y.; Masaphy, S.; Root, R.A.; Rytwo, G. Immobilization of Rhus vernicifera laccase on sepiolite; effect of chitosan and copper modification on laccase adsorption and activity. Appl. Clay Sci. 2018, 152, 143–147. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Zhang, J.; Xu, J. Effect of sorbitol content on microstructure and thermal properties of chitosan films. Int. J. Biol. Macromol. 2018, 119, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.; El Mehtedi, M.; Bottegoni, C.; Gigante, A. Physical properties imparted by genipin to chitosan for tissue regeneration with human stem cells: A review. Int. J. Biol. Macromol. 2016, 93, 1366–1381. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. The dramatic effect of small pH changes on the properties of chitosan hydrogels crosslinked with genipin. Carbohydr. Polym. 2015, 127, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-H.; Wu, S.-J.; Wu, J.-Y.; Wen, D.-Y.; Mi, F.-L. Preparation of fucoidan-shelled and genipin-crosslinked chitosan beads for antibacterial application. Carbohydr. Polym. 2015, 126, 97–107. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Kamala-Kannan, S.; Cho, M.; Kim, J.S.; Hadibarata, T.; Salim, M.R.; Oh, B.-T. Laccase immobilization on cellulose nanofiber: The catalytic efficiency and recyclic application for simulated dye effluent treatment. J. Mol. Catal. B Enzym. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, D.; Wei, Y.; Sarhan, A. Preparation and characterization of magnetic chelating resin based on chitosan for adsorption of Cu(II), Co(II), and Ni(II) ions. React. Funct. Polym. 2010, 70, 257–266. [Google Scholar] [CrossRef]
- Lambert, J.B. Introduction to Organic Spectroscopy. In Introduction to Organic Spectroscopy; Lambert, J.B., Shurvell, H.F., Lightner, D.A., Cooks, R.G., Eds.; Macmillan Publishing Company: New York, NY, USA, 1987. [Google Scholar]
- Gámiz-González, M.; Correia, D.; Lanceros-Mendez, S.; Sencadas, V.; Ribelles, J.G.; Vidaurre, A. Kinetic study of thermal degradation of chitosan as a function of deacetylation degree. Carbohydr. Polym. 2017, 167, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Zohuriaan, M.; Shokrolahi, F. Thermal studies on natural and modified gums. Polym. Test. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Peniche-Covas, C.; Argüelles-Monal, W.; Román, J.S. A kinetic study of the thermal degradation of chitosan and a mercaptan derivative of chitosan. Polym. Degrad. Stab. 1993, 39, 21–28. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, C.; Ni, H.; Zhu, Y.; Jiang, Z.; Xiao, A. β-Agarase immobilized on tannic acid-modified Fe3O4 nanoparticles for efficient preparation of bioactive neoagaro-oligosaccharide. Food Chem. 2019, 272, 586–595. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, F.; Lu, Y.; Ge, W.; Zhang, M.; Yue, B. Immobilization of pectinase from Penicillium oxalicum F67 onto magnetic cornstarch microspheres: Characterization and application in juice production. J. Mol. Catal. B Enzym. 2013, 97, 137–143. [Google Scholar] [CrossRef]
- Ji, Q.; Wang, B.; Tan, J.; Zhu, L.; Li, L. Immobilized multienzymatic systems for catalysis of cascade reactions. Process. Biochem. 2016, 51, 1193–1203. [Google Scholar] [CrossRef]
- Atacan, K.; Özacar, M. Characterization and immobilization of trypsin on tannic acid modified Fe3O4 nanoparticles. Colloid Surf. B Biointerfaces 2015, 128, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fu, G.; Liu, C.; McClements, D.J.; Wan, Y.; Wang, S.; Liu, T. Tannase immobilisation by amino-functionalised magnetic Fe3O4-chitosan nanoparticles and its application in tea infusion. Int. J. Biol. Macromol. 2018, 114, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Venezia, V.; Sannino, F.; Costantini, A.; Silvestri, B.; Cimino, S.; Califano, V. Mesoporous silica nanoparticles for β-glucosidase immobilization by templating with a green material: Tannic acid. Microporous Mesoporous Mater. 2020, 302, 110203. [Google Scholar] [CrossRef]
- Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent Immobilization of β-Glucosidase into Mesoporous Silica Nanoparticles from Anhydrous Acetone Enhances Its Catalytic Performance. Nanomaterials 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Figueira, J.A.; Sato, H.H.; Fernandes, P. Establishing the Feasibility of Using β-Glucosidase Entrapped in Lentikats and in Sol–Gel Supports for Cellobiose Hydrolysis. J. Agric. Food Chem. 2013, 61, 626–634. [Google Scholar] [CrossRef]
- Verma, M.L.; Rajkhowa, R.; Wang, X.; Barrow, C.J.; Puri, M. Exploring novel ultrafine Eri silk bioscaffold for enzyme stabilisation in cellobiose hydrolysis. Bioresour. Technol. 2013, 145, 302–306. [Google Scholar] [CrossRef]
- Bursal, E.; Koeksal, E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.). Food Res. Int. 2011, 44, 2217–2221. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Castiglioni, S.; Damiani, E.; Astolfi, P.; Carloni, P. Influence of steeping conditions (time, temperature, and particle size) on antioxidant properties and sensory attributes of some white and green teas. Int. J. Food Sci. Nutr. 2015, 66, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Kataki, M.S.; Kakoti, B.B.; Bhuyan, B.; Rajkumari, A.; Rajak, P. Garden rue inhibits the arachidonic acid pathway, scavenges free radicals, and elevates FRAP: Role in inflammation. Chin. J. Nat. Med. 2014, 12, 172–179. [Google Scholar] [CrossRef]
- Lu, M.-J.; Chen, C. Enzymatic modification by tannase increases the antioxidant activity of green tea. Food Res. Int. 2008, 41, 130–137. [Google Scholar] [CrossRef]
- Macedo, J.; Battestin, V.; Ribeiro, M. Increasing the antioxidant power of tea extracts by biotransformation of polyphenols. Food Chem. 2011, 126, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Almajano, M.P.; Carbó, R.; Jiménez, J.A.L.; Gordon, M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008, 108, 55–63. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Li, Y.; Cui, S.W.; Jin, Z. Elucidation of structural difference in theaflavins for modulation of starch digestion. J. Funct. Foods 2013, 5, 2024–2029. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Li, Y.; Cui, S.W.; Zhang, T. Structure elucidation of catechins for modulation of starch digestion. LWT 2014, 57, 188–193. [Google Scholar] [CrossRef]
- Yang, X.; Kong, F. Effects of tea polyphenols and different teas on pancreatic α-amylase activity in vitro. LWT 2016, 66, 232–238. [Google Scholar] [CrossRef]
- Qu, F.; Zeng, W.; Tong, X.; Feng, W.; Chen, Y.; Ni, D. The new insight into the influence of fermentation temperature on quality and bioactivities of black tea. LWT 2020, 117, 108646. [Google Scholar] [CrossRef]
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K. α-Glucosidase Inhibitory Profile of Catechins and Theaflavins. J. Agric. Food. Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; A Vattem, D.; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Bhat, T.; Dawra, R. A Spectrophotometric Method for Assay of Tannase Using Rhodanine. Anal. Biochem. 2000, 279, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Shuyuan, L.; Zeyi, A.; Fengfeng, Q.; Yuqiong, C.; Dejiang, N. Effect of steeping temperature on antioxidant and inhibitory activities of green tea extracts against α-amylase, α-glucosidase and intestinal glucose uptake. Food Chem. 2017, 234, 168–173. [Google Scholar]




 ) and GP–CS–tannase (
) and GP–CS–tannase (  ). (a) The optimal pH For Tannase was determined by performing an activity assay with four different buffers (50 mM) at pH 3.0–10.0, citrate buffer at pH 3.0–6.0, phosphate at pH 6.0–8.0, Tris-HCl buffer at pH 8.0–9.0, and Gly-NaOH buffer at pH 9.0–10.0. (b) The pH stability of Tannase was determined during incubation at different pH values and 4 °C for 24 h. (c) The determination of the optimum temperatures for free and immobilized tannase at different temperatures and at pH 5.0 and 6.0. (d–f) The thermal stability of free and immobilized tannase was determined after incubation at 50 °C, 60 °C, and 70 °C. The half-life was obtained by fitting with GraphPad Prism software. All data represent the mean ± standard deviation of triplicate measurements.
). (a) The optimal pH For Tannase was determined by performing an activity assay with four different buffers (50 mM) at pH 3.0–10.0, citrate buffer at pH 3.0–6.0, phosphate at pH 6.0–8.0, Tris-HCl buffer at pH 8.0–9.0, and Gly-NaOH buffer at pH 9.0–10.0. (b) The pH stability of Tannase was determined during incubation at different pH values and 4 °C for 24 h. (c) The determination of the optimum temperatures for free and immobilized tannase at different temperatures and at pH 5.0 and 6.0. (d–f) The thermal stability of free and immobilized tannase was determined after incubation at 50 °C, 60 °C, and 70 °C. The half-life was obtained by fitting with GraphPad Prism software. All data represent the mean ± standard deviation of triplicate measurements.
 ) and GP–CS–tannase (
) and GP–CS–tannase (  ). (a) The optimal pH For Tannase was determined by performing an activity assay with four different buffers (50 mM) at pH 3.0–10.0, citrate buffer at pH 3.0–6.0, phosphate at pH 6.0–8.0, Tris-HCl buffer at pH 8.0–9.0, and Gly-NaOH buffer at pH 9.0–10.0. (b) The pH stability of Tannase was determined during incubation at different pH values and 4 °C for 24 h. (c) The determination of the optimum temperatures for free and immobilized tannase at different temperatures and at pH 5.0 and 6.0. (d–f) The thermal stability of free and immobilized tannase was determined after incubation at 50 °C, 60 °C, and 70 °C. The half-life was obtained by fitting with GraphPad Prism software. All data represent the mean ± standard deviation of triplicate measurements.
). (a) The optimal pH For Tannase was determined by performing an activity assay with four different buffers (50 mM) at pH 3.0–10.0, citrate buffer at pH 3.0–6.0, phosphate at pH 6.0–8.0, Tris-HCl buffer at pH 8.0–9.0, and Gly-NaOH buffer at pH 9.0–10.0. (b) The pH stability of Tannase was determined during incubation at different pH values and 4 °C for 24 h. (c) The determination of the optimum temperatures for free and immobilized tannase at different temperatures and at pH 5.0 and 6.0. (d–f) The thermal stability of free and immobilized tannase was determined after incubation at 50 °C, 60 °C, and 70 °C. The half-life was obtained by fitting with GraphPad Prism software. All data represent the mean ± standard deviation of triplicate measurements.



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Chen, P.-X.; Xiao, Q.; Yang, Q.-M.; Weng, H.-F.; Zhang, Y.-H.; Xiao, A.-F. Chitosan Activated with Genipin: A Nontoxic Natural Carrier for Tannase Immobilization and Its Application in Enhancing Biological Activities of Tea Extract. Mar. Drugs 2021, 19, 166. https://doi.org/10.3390/md19030166
Wang C, Chen P-X, Xiao Q, Yang Q-M, Weng H-F, Zhang Y-H, Xiao A-F. Chitosan Activated with Genipin: A Nontoxic Natural Carrier for Tannase Immobilization and Its Application in Enhancing Biological Activities of Tea Extract. Marine Drugs. 2021; 19(3):166. https://doi.org/10.3390/md19030166
Chicago/Turabian StyleWang, Chi, Pei-Xu Chen, Qiong Xiao, Qiu-Ming Yang, Hui-Fen Weng, Yong-Hui Zhang, and An-Feng Xiao. 2021. "Chitosan Activated with Genipin: A Nontoxic Natural Carrier for Tannase Immobilization and Its Application in Enhancing Biological Activities of Tea Extract" Marine Drugs 19, no. 3: 166. https://doi.org/10.3390/md19030166