Synthesis of Novel Chitin Derivatives Bearing Amino Groups and Evaluation of Their Antifungal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chitin Derivatives Preparation and Characterization
2.1.1. Synthesis of Chitin Derivatives and Elemental Analysis
2.1.2. FT-IR Spectra
2.1.3. Solid-State 13C NMR Analysis
2.1.4. X-ray Diffraction (XRD) Analysis
2.1.5. Scanning Electron Microscopy (SEM) Analysis
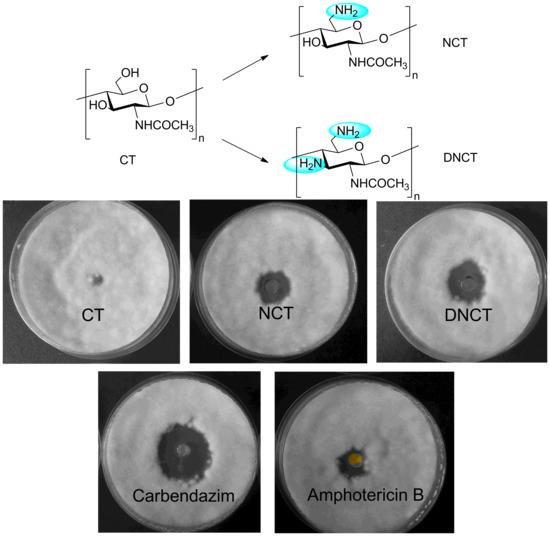
2.2. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Synthesis of Chitin Derivatives
3.3.1. Tosylation of Chitin [25]
3.3.2. Azidation of Tosyl Chitin
3.3.3. Reduction of Azide Group
3.4. Evaluation of Antifungal Activity in Vitro
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ai, H.; Wang, F.; Xia, Y.; Chen, X.; Lei, C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012, 132, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Ho, T.C.; Chae, S.J.; Cho, Y.J.; Park, J.S.; Lee, H.J.; Chun, B.S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, M.R.; Fathi, M. Production and characterization of chitin nanocrystals from prawn shell and their application for stabilization of Pickering emulsions. Food Hydrocolloid 2018, 82, 338–345. [Google Scholar] [CrossRef]
- Casteleijn, M.G.; Richardson, D.; Parkkila, P.; Granqvist, N.; Urtti, A.; Viitala, T. Spin coated chitin films for biosensors and its analysis are dependent on chitin-surface interactions. Colloids Surf. A 2018, 539, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Baran, T.; Erdogan, S.; Mentes, A.; Ozusaglam, M.A.; Cakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C 2014, 45, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Sayari, N.; Sila, A.; Abdelmalek, B.E.; Abdallah, R.B.; Ellouz-Chaabouni, S.; Bougatef, A.; Balti, R. Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities. Int. J. Biol. Macromol. 2016, 87, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar. Drugs 2011, 9, 1510–1533. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.R.; Elella, M.H.; Sabaa, M.W. Cytotoxicity and metal ions removal using antibacterial biodegradable hydrogels based on N-quaternized chitosan/poly(acrylic acid). Int. J. Biol. Macromol. 2017, 98, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, H.; Qiu, M.; Geng, X.; Yang, R.; Li, J.; Zhang, C. Antibacterial activity evaluation of quaternary chitin against Escherichia coli and Staphylococcus aureus. Int. J. Biol. Macromol. 2013, 52, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Morkaew, T.; Pinyakong, O.; Tachaboonyakiat, W. Structural effect of quaternary ammonium chitin derivatives on their bactericidal activity and specificity. Int. J. Biol. Macromol. 2017, 101, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, Y.; Wang, L.; Guo, Y.; Tan, H. Synthesis of chitosan C6-substituted cyclodextrin derivatives with tosyl-chitin as the intermediate precursor. J. Appl. Polym. Sci. 2012, 125, E378–E383. [Google Scholar] [CrossRef]
- Chirachanchai, S.; Lertworasirikul, A.; Tachaboonyakiat, W. Carbaryl insecticide conjugation onto chitosan via iodochitosan and chitosan carbonyl imidazolide precursors. Carbohydr. Polym. 2001, 46, 19–27. [Google Scholar] [CrossRef]
- Khan, F.I.; Rahman, S.; Queen, A.; Ahamad, S.; Ali, S.; Kim, J.; Hassan, M.I. Implications of molecular diversity of chitin and its derivatives. Appl. Microbiol. Biotechnol. 2017, 101, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Meng, X.; Xing, R.; Liu, S.; Chen, X.; Qin, Y.; Yu, H.; Li, P. Design, synthesis and antimicrobial activity of 6-N-substituted chitosan derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 4548–4551. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Ren, J.; Wang, P.; Dong, F.; Feng, Y.; Peng, D.; Guo, Z. Synthesis and antifungal properties of 6-amino-6-deoxyinulin, a kind of precursors for facile chemical modifications of inulin. Carbohydr. Polym. 2012, 87, 1744–1748. [Google Scholar] [CrossRef] [Green Version]
- Luan, F.; Li, Q.; Tan, W.; Wei, L.; Zhang, J.; Dong, F.; Gu, G.; Guo, Z. The evaluation of antioxidant and antifungal properties of 6-amino-6-deoxychitosan in vitro. Int. J. Biol. Macromol. 2018, 107, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Raina-Fulton, R. New Trends in Pesticide Residue Analysis in Cereals, Nutraceuticals, Baby Foods, and Related Processed Consumer Products. J. AOAC Int. 2015, 98, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Lukowska-Chojnacka, E.; Mierzejewska, J.; Milner-Krawczyk, M.; Bondaryk, M.; Staniszewska, M. Synthesis of novel tetrazole derivatives and evaluation of their antifungal activity. Bioorg. Med. Chem. 2016, 24, 6058–6065. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crops Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Luan, F.; Yin, X.; Dong, F.; Li, Q.; Guo, Z. Synthesis of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate for Antifungal and Antibacterial Activities. Polymer 2018, 10, 530. [Google Scholar] [CrossRef]
- Munro, N.H.; Hanton, L.R.; Moratti, S.C.; Robinson, B.H. Preparation and graft copolymerisation of thiolated β-chitin and chitosan derivatives. Carbohydr. Polym. 2009, 78, 137–145. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Shanmugam, V.; Shanmugam, A. Extraction, characterization and antioxidant property of chitosan from cuttlebone Sepia kobiensis (Hoyle 1885). Int. J. Biol. Macromol. 2014, 64, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.D.; Abeyratne, C.; Bangamuwa, O.M.; Ludowyke, N.; Dahanayake, D.; Gunasekara, S.; de Silva, N.; de Silva, R.M.; de Silva, K.M.N. In-situ formation of supramolecular aggregates between chitin nanofibers and silver nanoparticles. Carbohydr. Polym. 2017, 173, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M.; Hrdina, R.; Abdel-Mohsen, A.M.; Fouda, M.M.; Soliman, A.Y.; Mohamed, F.K.; Mohsin, K.; Pinto, T.D. Chitin and chitosan from Brazilian Atlantic Coast: Isolation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2015, 80, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Shahzadi, L.; Mahmood, N.; Siddiqi, S.A.; Rauf, A.; Manzoor, F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. A new synthetic methodology for the preparation of biocompatible and organo-soluble barbituric- and thiobarbituric acid based chitosan derivatives for biomedical applications. Mater. Sci. Eng. C 2016, 66, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and in vitro antioxidant activities of 6-amino-6-deoxychitosan and its sulfonated derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Guo, Z. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. Int. J. Biol. Macromol. 2014, 70, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef] [PubMed]





| Monosubstituted Chitin Derivatives | Disubstituted Chitin Derivatives | ||
|---|---|---|---|
| Full Name | Abbreviations | Full Name | Abbreviations |
| 6-tosyl-chitin | TCT | 3,6-ditosyl-chitin | DTCT |
| 6-azido-chitin | ACT | 3,6-diazido-chitin | DACT |
| 6-amino-chitin | NCT | 3,6-diamino-chitin | DNCT |
| Sample | Found (%) | DS | Formula | |||
|---|---|---|---|---|---|---|
| C | H | N | S | |||
| CT | 45.2 | 7.2 | 6.6 | - | - | C8H13NO5 |
| TCT | 50.2 | 6.6 | 4.0 | 8.3 | 0.93 | (C15H19NO7S)0.93(CT)0.07 |
| DTCT | 50.3 | 13.9 | 3.6 | 10.0 | 1.2 | (C15H19NO7S)0.8(C22H25NO9S2)0.2 |
| ACT | 43.3 | 5.3 | 21.9 | - | 0.97 | (TCT)0.1 (C8H12N4O4)0.9 |
| DACT | 40.4 | 4.7 | 21.3 | - | 1.14 | (DTCT)0.03(C8H12N4O4)0.78 (C8H11N7O3)0.18 |
| NCT | 47.0 | 6.8 | 15.0 | - | 0.96 | (ACT)0.04 (C8H14N2O4)0.87 |
| DNCT | 45.5 | 6.4 | 13.3 | - | 1.11 | (DACT)0.03 (C8H14N2O4)0.76(C8H15N3O3)0.17 |
| Sample | Inhibition Zone Diameter Against Various Fungi (mm) a | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| Carbendazim | 24.3 ± 1.3 | 19.9 ± 0.9 | 16.3 ± 0.5 | 27.8 ± 1.1 | 18.6 ± 0.4 | 21.8 ± 1.0 |
| Amphotericin B | 16.1 ± 1.0 | 14.2 ± 0.4 | 8.0 ± 0.8 | 18.9 ± 1.8 | 9.6 ± 0.5 | 15.7 ± 0.7 |
| CT | 5.3 ± 0.1 | 5.5 ± 0.2 | 5.3 ± 0.1 | 5.8 ± 0.2 | 5.8 ± 0.2 | 6.1 ± 0.2 |
| NCT | 16.3 ± 1.3 * | 11.2 ± 0.5 ** | 13.1 ± 0.9 * | 14.2 ± 1.0 * | 12.4 ± 0.2 ** | 14.9 ± 0.6 ** |
| DNCT | 20.4 ± 1.7 ** | 11.4 ± 0.2 ** | 14.0 ± 0.5 ** | 15.6 ± 1.5 * | 14.2 ± 0.5 ** | 16.3 ± 1.3 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Luan, F.; Li, Q.; Gu, G.; Dong, F.; Guo, Z. Synthesis of Novel Chitin Derivatives Bearing Amino Groups and Evaluation of Their Antifungal Activity. Mar. Drugs 2018, 16, 380. https://doi.org/10.3390/md16100380
Zhang J, Luan F, Li Q, Gu G, Dong F, Guo Z. Synthesis of Novel Chitin Derivatives Bearing Amino Groups and Evaluation of Their Antifungal Activity. Marine Drugs. 2018; 16(10):380. https://doi.org/10.3390/md16100380
Chicago/Turabian StyleZhang, Jingjing, Fang Luan, Qing Li, Guodong Gu, Fang Dong, and Zhanyong Guo. 2018. "Synthesis of Novel Chitin Derivatives Bearing Amino Groups and Evaluation of Their Antifungal Activity" Marine Drugs 16, no. 10: 380. https://doi.org/10.3390/md16100380