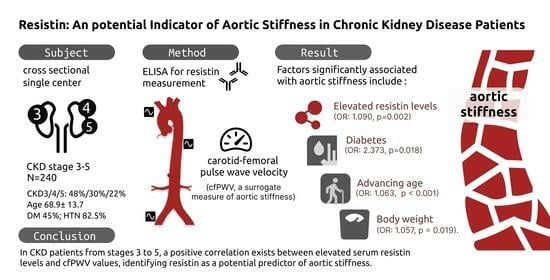
Resistin: A Potential Indicator of Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrollees
2.2. Physical Measurements
2.3. Biochemical Investigations
2.4. Carotid–Femoral Pulse Wave Velocity Measurements
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of This Study
3.2. Resistin Level Is Associated with Aortic Stiffness in Patients with CKD
3.3. Correlation between Resistin Levels and Clinical and Biochemical Parameters in CKD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.L.; Lima, J.A.C.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Cecelja, M.; Chowienczyk, P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc. Dis. 2012, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Faconti, L.; Nanino, E.; Mills, C.E.; Cruickshank, K.J. Do arterial stiffness and wave reflection underlie cardiovascular risk in ethnic minorities? JRSM Cardiovasc. Dis. 2016, 5, 2048004016661679. [Google Scholar] [CrossRef]
- Alani, H.; Tamimi, A.; Tamimi, N. Cardiovascular co-morbidity in chronic kidney disease: Current knowledge and future research needs. World J. Nephrol. 2014, 3, 156–168. [Google Scholar] [CrossRef]
- Lioufas, N.; Hawley, C.M.; Cameron, J.D.; Toussaint, N.D. Chronic kidney disease and pulse wave velocity: A narrative review. Int. J. Hypertens. 2019, 2019, 9189362. [Google Scholar] [CrossRef]
- Garnier, A.S.; Briet, M. Arterial stiffness and chronic kidney disease. Pulse 2015, 3, 229–241. [Google Scholar] [CrossRef]
- Jamaluddin, M.S.; Weakley, S.M.; Yao, Q.; Chen, C. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 2012, 165, 622–632. [Google Scholar] [CrossRef]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef]
- Wang, J.H.; Lee, C.J.; Yang, C.F.; Chen, Y.C.; Hsu, B.G. Serum resistin as an independent marker of aortic stiffness in patients with coronary artery disease. PLoS ONE 2017, 12, e0183123. [Google Scholar] [CrossRef]
- Romejko, K.; Rymarz, A.; Szamotulska, K.; Bartoszewicz, Z.; Rozmyslowicz, T.; Niemczyk, S. Resistin Contribution to Cardiovascular Risk in Chronic Kidney Disease Male Patients. Cells 2023, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42 (Suppl. S3), S1–S201. [Google Scholar] [CrossRef]
- Chi, P.J.; Liou, H.H.; Hsu, B.G.; Tasi, J.P. Relationship between resistin and mortality in maintenance hemodialysis patients. Clin. Nephrol. 2016, 86, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.G.; Lee, C.J.; Yang, C.F.; Chen, Y.C.; Wang, J.H. High serum resistin levels are associated with peripheral artery disease in the hypertensive patients. BMC Cardiovasc. Disord. 2017, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Lopes, L.R.; Ribeiro, S.M.L.T.; Figueiredo, V.P.; Leite, A.L.J.; Nicolato, R.L.C.; Gomes, J.A.E.; de Oliveira, F.L.P.; Talvani, A. The overweight increases circulating inflammatory mediators commonly associated with obesity in young individuals. Cytokine 2018, 110, 169–173. [Google Scholar] [CrossRef]
- Rae, C.; Graham, A. Human resistin promotes macrophage lipid accumulation. Diabetologia 2006, 49, 1112–1114. [Google Scholar] [CrossRef]
- Sethi, S.; Rivera, O.; Oliveros, R.; Chilton, R. Aortic stiffness: Pathophysiology, clinical implications, and approach to treatment. Integr. Blood Press. Control. 2014, 7, 29–34. [Google Scholar] [CrossRef] [PubMed]
- van Sloten, T.T.; Schram, M.T.; van den Hurk, K.; Dekker, J.M.; Nijpels, G.; Henry, R.M.; Stehouwer, C.D. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: The Hoorn study. J. Am. Coll. Cardiol. 2014, 63, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Spadaccio, C.; Lusini, M.; Ulianich, L.; Chello, M.; Nappi, F. Basic and clinical research against advanced glycation end products (AGEs): New compounds to tackle cardiovascular disease and diabetic complications. Recent Adv. Cardiovasc. Drug Discov. 2015, 10, 10–33. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Du Yan, S.; Stern, D.M. The dark side of glucose. Nat. Med. 1995, 1, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Okada, Y.; Tanikawa, R.; Tanaka, Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J. Vasc. Res. 2009, 46, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Li, W.B.; Zhao, J.; Liu, L.; Wang, Z.H.; Han, L.; Zhong, M.; Zhang, Y.; Zhang, W.; Tang, M.X. Silencing of activin receptor-like kinase 7 alleviates aortic stiffness in type 2 diabetic rats. Acta Diabetol. 2015, 52, 717–726. [Google Scholar] [CrossRef]
- Wohlfahrt, P.; Somers, V.K.; Sochor, O.; Kullo, I.; Jean, N.; Lopez-Jimenez, F. Influence of body fatness distribution and total lean mass on aortic stiffness in nonobese individuals. Am. J. Hypertens. 2014, 28, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Martos-Moreno, G.A.; Barrios, V.; Argente, J. Normative data for adiponectin, resistin, interleukin 6, and leptin/receptor ratio in a healthy Spanish pediatric population: Relationship with sex steroids. Eur. J. Endocrinol. 2006, 155, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef]
- Lim, C.C.; Teo, B.W.; Ong, P.G.; Cheung, C.Y.; Lim, S.C.; Chow, K.Y.; Meng, C.C.; Lee, J.; Tai, E.S.; Wong, T.Y.; et al. Chronic kidney disease, cardiovascular disease and mortality: A prospective cohort study in a multi-ethnic Asian population. Eur. J. Prev. Cardiol. 2015, 22, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Mattace-Raso, F.U.; Hoorn, E.J.; Uitterlinden, A.G.; Hofman, A.; Ikram, M.A.; Franco, O.H.; Dehghan, A. Arterial stiffness and decline in kidney function. Clin. J. Am. Soc. Nephrol. 2015, 10, 2190–2197. [Google Scholar] [CrossRef]
- Huang, N.; Foster, M.C.; Mitchell, G.F.; Andresdottir, M.B.; Eiriksdottir, G.; Gudmundsdottir, H.; Harris, T.B.; Launer, L.J.; Palsson, R.; Gudnason, V.; et al. Aortic stiffness and change in glomerular filtration rate and albuminuria in older people. Nephrol. Dial. Transplant. 2017, 32, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Woodard, T.; Sigurdsson, S.; Gotal, J.D.; Torjesen, A.A.; Inker, L.A.; Aspelund, T.; Eiriksdottir, G.; Gudnason, V.; Harris, T.B.; Launer, L.J.; et al. Mediation analysis of aortic stiffness and renal microvascular function. J. Am. Soc. Nephrol. 2015, 26, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Rzepa, Ł.; Peller, M.; Eyileten, C.; Rosiak, M.; Kondracka, A.; Mirowska-Guzel, D.; Opolski, G.; Filipiak, K.J.; Postuła, M.; Kapłon-Cieslicka, A. Resistin is associated with inflammation and renal function, but not with insulin resistance in type 2 diabetes. Horm. Metab. Res. 2021, 53, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Eguchi, K.; O’Rourke, M.F.; Ishikawa, J.; Miyashita, H.; Shimada, K.; Kario, K. Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients. Hypertension 2009, 54, 716–723. [Google Scholar] [CrossRef]
- Skoczylas, A.; Piecha, G.; Więcek, A. Effects of antihypertensive treatment on plasma apelin, resistin, and visfatin concentrations. Pol. Arch. Med. Wewn. 2016, 126, 243–253. [Google Scholar] [CrossRef] [PubMed]
| Clinical Attributes | All Patients (n = 240) | Non-Aortic Stiffness Group (n = 152) | Aortic Stiffness Group (n = 88) | p-Value |
|---|---|---|---|---|
| Age (years) | 68.85 ± 13.66 | 66.18 ± 14.10 | 73.47 ± 11.55 | <0.001 * |
| Height (cm) | 158.63 ± 8.56 | 158.11 ± 8.30 | 159.54 ± 8.97 | 0.211 |
| Body weight (kg) | 65.74 ± 14.26 | 63.45 ± 13.31 | 69.71 ± 15.05 | 0.001 * |
| BMI (kg/m2) | 26.02 ± 4.65 | 25.31 ± 4.55 | 27.24 ± 4.57 | 0.002 * |
| Body fat mass (%) | 28.90 ± 8.86 | 27.65 ± 9.07 | 31.22 ± 8.06 | 0.002 * |
| cfPWV (m/s) | 9.05 (7.20–11.28) | 7.70 (6.53–8.90) | 12.45 (10.83–14.45) | <0.001 * |
| SBP (mmHg) | 149.98 ± 24.90 | 145.24 ± 24.18 | 158.17 ± 24.11 | <0.001 * |
| DBP (mmHg) | 84.35 ± 13.37 | 83.01 ± 12.74 | 86.68 ± 14.178 | 0.004 * |
| TCH (mg/dL) | 159.91 ± 41.63 | 160.08 ± 44.59 | 159.63 ± 36.21 | 0.935 |
| Triglyceride (mg/dL) | 122.0 (91.00–170.00) | 118.50 (87.25–164.00) | 135.50 (95.75–188.00) | 0.063 |
| Fasting glucose (mg/dL) | 99.00 (90.00–126.75) | 97.00 (89.00–124.50) | 107.00 (94.25–134.50) | 0.009 * |
| BUN (mg/dL) | 33.50 (24.25–50.00) | 33.00 (23.25–51.75) | 35.00 (26.25–47.00) | 0.410 |
| Creatinine (mg/dL) | 1.95 (1.40–2.90) | 1.90 (1.40–2.88) | 2.05 (1.50–3.05) | 0.346 |
| eGFR (mL/min) | 30.80 ± 15.56 | 31.90 ± 16.26 | 28.92 ± 14.15 | 0.154 |
| Total calcium (mg/dL) | 8.88 (8.60–9.24) | 8.86 (8.56–9.28) | 8.96 (8.68–9.20) | 0.594 |
| Phosphorus (mg/dL) | 3.70 (3.20–4.28) | 3.70 (3.23–4.30) | 3.70 (3.10–4.20) | 0.433 |
| Resistin (ng/mL) | 5.17 (3.31–8.21) | 7.39 (5.89–11.73) | 10.70 (7.14–18.85) | <0.001 * |
| Female, n (%) | 110 (45.8) | 76 (50.0) | 34 (38.6) | 0.089 |
| Diabetes mellitus, n (%) | 107 (44.6) | 59 (38.8) | 48 (54.5) | 0.018 * |
| Hypertension, n (%) | 198 (82.5) | 127 (83.6) | 71 (80.7) | 0.573 |
| Glomerulonephritis, n (%) | 53 (22.1) | 39 (25.7) | 14 (15.9) | 0.079 |
| CKD stage 3, n (%) | 116 (48.3) | 77 (50.7) | 39 (44.3) | 0.538 |
| CKD stage 4, n (%) | 72 (30.0) | 42 (27.6) | 30 (34.1) | |
| CKD stage 5, n (%) | 52 (21.7) | 33 (21.7) | 19 (21.9) |
| Variables | Odds Ratio | 95% C.I. | p-Value |
|---|---|---|---|
| Resistin, 1 ng/mL | 1.090 | 1.032–1.152 | 0.002 * |
| Age, 1 year | 1.063 | 1.031–1.095 | <0.001 * |
| Diabetes mellitus, present | 2.373 | 1.161–4.852 | 0.018 * |
| Body weight, 1 kg | 1.057 | 1.009–1.107 | 0.019 * |
| Systolic blood pressure, 1 mmHg | 1.015 | 0.995–1.036 | 0.140 |
| Diastolic blood pressure, 1 mmHg | 1.015 | 0.977–1.054 | 0.448 |
| Body fat mass, 1% | 1.032 | 0.987–1.079 | 0.170 |
| Body mass index, 1 kg/m2 | 0.884 | 0.759–1.029 | 0.112 |
| Fasting glucose, 1 mg/dL | 1.001 | 0.993–1.008 | 0.856 |
| Variables | Log-Resistin (ng/mL) | ||||
|---|---|---|---|---|---|
| Simple Regression | Multivariable Regression | ||||
| r | p-Value | Beta | Adjusted R2 Change | p-Value | |
| Female | 0.009 | 0.885 | — | — | — |
| Diabetes mellitus | −0.019 | 0.771 | — | — | — |
| Hypertension | −0.075 | 0.245 | — | — | — |
| Glomerulonephritis | 0.032 | 0.619 | — | — | — |
| Age (years) | 0.120 | 0.064 | — | — | — |
| Height (cm) | −0.026 | 0.686 | — | — | — |
| Body weight (kg) | 0.244 | <0.001 * | — | — | — |
| BMI (kg/m2) | 0.305 | <0.001 * | — | — | — |
| Body fat mass (%) | 0.442 | <0.001 * | 0.401 | 0.192 | <0.001 * |
| Log-cfPWV (m/s) | 0.249 | <0.001 * | 0.204 | 0.031 | <0.001 * |
| SBP (mmHg) | 0.124 | 0.054 | — | — | — |
| DBP (mmHg) | 0.102 | 0.115 | — | — | — |
| TCH (mg/dL) | −0.023 | 0.719 | — | — | — |
| Log-Triglyceride (mg/dL) | −0.134 | 0.037 * | −0.130 | 0.013 | 0.024 * |
| Log-Glucose (mg/dL) | 0.058 | 0.367 | — | — | — |
| Log-BUN (mg/dL) | 0.027 | 0.679 | — | — | — |
| Log-Creatinine (mg/dL) | 0.024 | 0.710 | — | — | — |
| eGFR (mL/min) | −0.051 | 0.431 | — | — | — |
| Log-Calcium (mg/dL) | 0.012 | 0.852 | — | — | — |
| Log-Phosphorus (mg/dL) | −0.053 | 0.410 | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-H.; Chen, M.-S.; Wang, C.-H.; Lai, Y.-H.; Lin, Y.-L.; Hsu, B.-G. Resistin: A Potential Indicator of Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients. Medicina 2023, 59, 1652. https://doi.org/10.3390/medicina59091652
Kuo C-H, Chen M-S, Wang C-H, Lai Y-H, Lin Y-L, Hsu B-G. Resistin: A Potential Indicator of Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients. Medicina. 2023; 59(9):1652. https://doi.org/10.3390/medicina59091652
Chicago/Turabian StyleKuo, Chiu-Huang, Min-Shuo Chen, Chih-Hsien Wang, Yu-Hsien Lai, Yu-Li Lin, and Bang-Gee Hsu. 2023. "Resistin: A Potential Indicator of Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients" Medicina 59, no. 9: 1652. https://doi.org/10.3390/medicina59091652