Autologous Platelet-Rich Plasma (PRP) Efficacy on Endometrial Thickness and Infertility: A Single-Centre Experience from Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
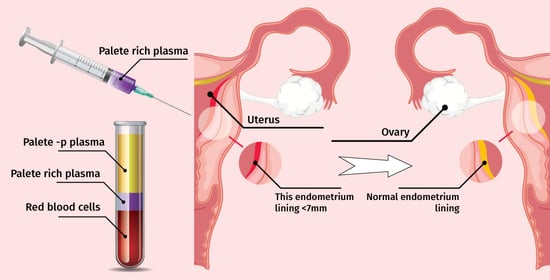
2.2. Endometrial Preparation
2.3. PRP Preparation and Infusion
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Population
3.2. Parameters of the Group before the Infusion with PRP
3.3. Parameters of the Group after PRP Treatment
3.4. Endometrial Thickness According to Age of Patients
3.5. Comparations between Group before and after PRP Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, K.E.; Hartman, M.; Hartman, A. Management of thin endometrium in assisted reproduction: A clinical practice guideline from the Canadian Fertility and Andrology Society. Reprod. Biomed. Online 2019, 39, 49–62. [Google Scholar] [CrossRef] [PubMed]
- The, W.T.; McBain, J.; Rogers, P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J. Assist. Reprod. Genet. 2016, 33, 1419–1430. [Google Scholar]
- Wang, Y.; Zhu, Y.; Sun, Y.; Di, W.; Qiu, M.; Kuang, Y.; Shen, H. Ideal embryo transfer position and endometrial thickness in IVF embryo transfer treatment. Int. J. Gynecol. Obstet. 2018, 143, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Mettler, L.; Jain, S.; Meshram, S.; Günther, V.; Alkatout, I. Management of a Thin Endometrium by Hysteroscopic Instillation of Platelet-Rich Plasma Into The Endomyometrial Junction: A Pilot Study. J. Clin. Med. 2020, 9, 2795. [Google Scholar] [CrossRef]
- Takasaki, A.; Tamura, H.; Miwa, I.; Taketani, T.; Shimamura, K.; Sugino, N. Endometrial growth and uterine blood flow: A pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil. Steril. 2010, 93, 1851–1858. [Google Scholar] [CrossRef]
- Roohaninasab, M.A.; Ghassemi, M.; Sadeghzadeh-Bazargan, A.; Behrangi, E.; Najar Nobari, N. Systematic review of platelet-rich plasma in treating alopecia: Focusing on efficacy, safety, and therapeutic durability. Dermatol. Ther. 2021, 34, e14768. [Google Scholar] [CrossRef]
- Chamata, E.S.; Bartlett, E.L.; Weir, D.; Rohrich, R.J. Platelet-rich plasma: Evolving role in plastic surgery. Plast. Reconstr. Surg. 2021, 147, 219–230. [Google Scholar] [CrossRef]
- Davey, M.S.; Davey, M.G.; Hurley, E.T.; Cassidy, J.T.; Mullett, H.; McInerney, N.M.; Galbraith, J.G. Platelet-rich plasma in non-operative management of mild to moderate carpal tunnel syndrome—A systematic review & meta-analysis of short-term outcomes. J. Orthop. 2021, 25, 155–161. [Google Scholar]
- Mouanness, M.; Ali-Bynom, S.; Jackman, J.; Seckin, S.; Merhi, Z. Use of Intra-uterine Injection of Platelet-rich Plasma (PRP) for Endometrial Receptivity and Thickness: A Literature Review of the Mechanisms of Action. Reprod. Sci. 2021, 28, 1659–1670. [Google Scholar] [CrossRef]
- Ershadi, S.; Noori, N.; Dashipoor, A.; Ghasemi, M.; Shamsa, N. Evaluation of the Effect of Intrauterine Injection of Platelet-Rich Plasma on the Pregnancy Rate of Patients with a History of Implantation Failure in the Fertilization Cycle. J. Fam. Med. Prim. Care 2022, 11, 2162–2166. [Google Scholar]
- Zargar, M.; Pazhouhanfar, R.; Najafian, M.; Choghakabodi, P.M. Effects of Intrauterine Autologous Platelet-Rich Plasma Infusions on Outcomes in Women with Repetitive in Vitro Fertilization Failures: A Prospective Randomized Study. Clin. Exp. Obstetr. Gynecol. 2021, 48, 180–185. [Google Scholar]
- Jacobs, E.A.; Van Voorhis, B.; Kawwass, J.F.; Kondapalli, L.A.; Liu, K.; Dokras, A. Endometrial thickness: How thin is too thin? Fertil. Steril. 2022, 118, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Saravelos, S.H.; Wang, Q.; Xu, Y.; Li, T.-C.; Zhou, C. Endometrial Thickness as a Predictor of Pregnancy Outcomes in 10787 Fresh IVF-ICSI Cycles. Reprod. Biomed. Online 2016, 33, 197–205. [Google Scholar] [CrossRef]
- Yin, W.; Qi, X.; Zhang, Y.; Sheng, J.; Xu, Z.; Tao, S.; Xie, X.; Li, X.; Zhang, C. Advantages of Pure Platelet-Rich Plasma Compared with Leukocyte- and Platelet-Rich Plasma in Promoting Repair of Bone Defects. J. Transl. Med. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Rodeo, S.A. Platelet-Rich Plasma in Orthopaedic Surgery: A Critical Analysis Review. JBJS Rev. 2017, 5, e7. [Google Scholar] [CrossRef]
- Scully, D.; Naseem, K.M.; Matsakas, A. Platelet Biology in Regenerative Medicine of Skeletal Muscle. Acta Physiol. 2018, 223, e13071. [Google Scholar] [CrossRef]
- Zadehmodarres, S.; Salehpour, S.; Saharkhiz, N.; Nazari, L. Treatment of thin endometrium with autologous platelet-rich plasma: A pilot study. JBRA Assist. Reprod. 2017, 21, 54–56. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lin, W.Y.; Lin, C.J.; Sun, Y.C.; Chang, P.Y.; Wang, H.Y.; Lu, J.J.; Yeh, W.L.; Chiueh, T.S. Sustained or Higher Levels of Growth Factors in Platelet-Rich Plasma During 7-Day Storage. Clin. Chim. Acta 2018, 483, 89–93. [Google Scholar] [CrossRef]
- Hesseler, M.J.; Shyam, N. Platelet-Rich Plasma and Its Utility in Medical Dermatology: A Systematic Review. J. Am. Acad. Dermatol. 2019, 81, 834–846. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.; Wei, L.-N.; Pang, J.; Chen, J.; Liang, X. Autologous Platelet-Rich Plasma Infusion Improves Clinical Pregnancy Rate in Frozen Embryo Transfer Cycles for Women with Thin Endometrium. Medicine 2019, 98, e14062. [Google Scholar] [CrossRef]
- Xu, Y.; Hao, C.; Fang, J.; Liu, X.; Xue, P.; Miao, R. Intrauterine Perfusion of Autologous Platelet-Rich Plasma before Frozen-Thawed Embryo Transfer Improves the Clinical Pregnancy Rate of Women with Recurrent Implantation Failure. Front. Med. 2022, 9, 850002. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hosseini, S.; Sheibani, S.; Hosseinirad, H. The Effects of Autologous Platelet-Rich Plasma on Pregnancy Outcomes in Repeated Implantation Failure Patients Undergoing Frozen Embryo Transfer: A Randomized Controlled Trial. Reprod. Sci. 2022, 29, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Cakiroglu, Y.; Saltik, A.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Scott, R.T.; Tiras, B.; Seli, E. Effects of Intraovarian Injection of Autologous Platelet Rich Plasma on Ovarian Reserve and Ivf Outcome Parameters in Women with Primary Ovarian Insufficiency. Aging 2020, 12, 10211–10222. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Hsu, L.; Hsu, I.; Chiu, Y.-J.; Dorjee, S. Live Birth in Woman with Premature Ovarian Insufficiency Receiving Ovarian Administration of Platelet-Rich Plasma (Prp) in Combination with Gonadotropin: A Case Report. Front. Endocrinol. 2020, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Salehpour, S.; Hoseini, S.; Zadehmodarres, S.; Azargashb, E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: A double-blind RCT. Int. J. Reprod. Biomed. 2019, 17, 443–448. [Google Scholar] [CrossRef]
- Moraes, V.Y.; Lenza, M.; Tamaoki, M.J.; Faloppa, F.; Belloti, J.C. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst. Rev. 2014, 4, CD010071. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, J.; Chen, Y.; Wei, L.; Yang, X.; Shi, Y.; Liang, X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015, 8, 1286–1290. [Google Scholar] [PubMed]
- Hu, S.; Jin, Z.; Tang, Q. Effects of Intrauterine Infusion of Autologous Platelet-Rich Plasma in Women Undergoing Treatment with Assisted Reproductive Technology: A Meta-Analysis of Randomized Controlled Trials. Geburtshilfe Frauenheilkd 2022, 83, 453–462. [Google Scholar] [CrossRef]
- Anitua, E.; de la Fuente, M.; Ferrando, M.; Quintana, F.; Larreategui, Z.; Matorras, R.; Orive, G. Biological effects of plasma rich in growth factors (PRGF) on human endometrial fibroblasts. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 125–130. [Google Scholar] [CrossRef]
- Rolla, E. Endometriosis: Advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Research 2019, 8, 529. [Google Scholar] [CrossRef]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; Pacheco, I.D.C.; Amaral, R.J.F.C.D.; Granjeiro, J.M.; Borojevic, R. Platelet-Rich Plasma Preparation for Regenerative Medicine: Optimization and Quantification of Cytokines and Growth Factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Yang, X.; Xing, Y.; Que, W.; Yu, Z.; Gui, W.; Chen, Y.; Liu, X. Intrauterine Infusion of Leukocyte-Poor Platelet-Rich Plasma Is an Effective Therapeutic Protocol for Patients with Recurrent Implantation Failure: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 2823. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, D.J.; Méndez-Bolaina, E.; Trejo-Bahena, N.I. Platelet-Rich Plasma Peptides: Key for Regeneration. Int. J. Pept. 2012, 2012, 532519. [Google Scholar] [CrossRef]
- Sharara, F.I.; Lelea, L.L.; Rahman, S.; Klebanoff, J.S.; Moawad, G.N. A Narrative Review of Platelet-Rich Plasma (Prp) in Reproductive Medicine. Review. J. Assist. Reprod. Genet. 2021, 38, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Tehraninejad, E.S.; Kashani, N.G.; Hosseini, A.; Tarafdari, A. Autologous Platelet-Rich Plasma Infusion Does Not Improve Pregnancy Outcomes in Frozen Embryo Transfer Cycles in Women with History of Repeated Implantation Failure without Thin Endometrium. J. Obstet. Gynaecol. Res. 2021, 47, 147–151. [Google Scholar] [CrossRef]
- Dogra, Y.; Singh, N.; Vanamail, P. Autologous platelet-rich plasma optimizes endometrial thickness and pregnancy outcomes in women with refractory thin endometrium of varied aetiology during fresh and frozen-thawed embryo transfer cycles. JBRA Assist. Reprod. 2022, 26, 13–21. [Google Scholar] [CrossRef]
- Efendieva, Z.; Vishnyakova, P.; Apolikhina, I.; Artemova, D.; Butov, K.; Kalinina, E.; Fedorova, T.; Tregubova, A.; Asaturova, A.; Fatkhudinov, T.; et al. Hysteroscopic injections of autologous endometrial cells and platelet-rich plasma in patients with thin endometrium: A pilot randomized study. Sci. Rep. 2023, 13, 945. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hosseini, M.S.; Moghanjoughi, P.H. The Effects of Autologous Platelet-Rich Plasma in Repeated Implantation Failure: A Randomized Controlled Trial. Hum. Fertil. 2020, 23, 209–213. [Google Scholar] [CrossRef]
- Luncan, M.; Huniadi, A.; Bimbo-Szuhai, E.; Botea, M.; Zaha, I.; Stefan, L.; Beiusanu, C.; Romanescu, D.; Pallag, A.; Bodog, A.; et al. The effectiveness of intrauterine antibiotic infusion versus oral antibiotic therapy in the treatment of chronic endometritis in patients during IVF (in vitro fertilization) procedures. BMC Women’s Health 2022, 22, 529. [Google Scholar] [CrossRef]
- Strug, M.; Aghajanova, L. Making More Womb: Clinical Perspectives Supporting the Development and Utilization of Mesenchymal Stem Cell Therapy for Endometrial Regeneration and Infertility. J. Pers. Med. 2021, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Safdarian, L.; Aleyasin, A.; Aghahoseini, M.; Lak, P.; Mosa, S.H.; Sarvi, F.; Mahdavi, A. Efficacy of the Intrauterine Infusion of Platelet-Rich Plasma on Pregnancy Outcomes in Patients with Repeated Implantation Failure: A Randomized Control Trial. Int. J. Women’s Health Reprod. Sci. 2022, 10, 38–44. [Google Scholar] [CrossRef]
- Zamaniyan, M.; Peyvandi, S.; Gorji, H.H.; Moradi, S.; Jamal, J.; Aghmashhadi, F.Y.P.; Mohammadi, M.H. Effect of Platelet-Rich Plasma on Pregnancy Outcomes in Infertile Women with Recurrent Implantation Failure: A Randomized Controlled Trial. Gynecol. Endocrinol. 2021, 37, 141–145. [Google Scholar] [CrossRef]
- Noushin, M.A.; Ashraf, M.; Thunga, C.; Singh, S.; Singh, S.; Basheer, R.; Ashraf, R.; Jayaprakasan, K. A Comparative Evaluation of Subendometrial and Intrauterine Platelet-Rich Plasma Treatment for Women with Recurrent Implantation Failure. F S Sci. 2021, 2, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.; Arora, S. Platelet-Rich Plasma-Where Do We Stand Today? A Critical Narrative Review and Analysis. Dermatol. Ther. 2021, 34, e14343. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; El-Mazny, A.; Kamal, N.; Mahmoud, S.I.; Fouad, M.; El-Nassery, N.; Kotb, A.; Ragab, W.S.; Ogila, A.I.; Metwally, A.A.; et al. The value of platelet-rich plasma in women with previous implantation failure: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2023, 40, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Albazee, E.; Al-Rshoud, F.; Almahmoud, L.; Al Omari, B.; Alnifise, M.; Baradwan, S.; Abu-Zaid, A. Platelet-rich plasma for the management of intrauterine adhesions: A systematic review and meta-analysis of randomized controlled trials. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102276. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Allende, M.; de la Fuente, M.; Del Fabbro, M.; Alkhraisat, M.H. Efficacy of Platelet-Rich Plasma in Women with a History of Embryo Transfer Failure: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Bioengineering 2023, 10, 303. [Google Scholar] [CrossRef]

| Baseline Characteristics of the Group | Group MD ± SD |
|---|---|
| Age of patients (years) | 38.23 ± 4.42 |
| Environment | |
| Urban | 23 (45.09%) |
| Rural | 28 (54.91%) |
| BMI (kg/m2) | 26.1 ± 3.7 |
| Infertility duration (years) | 5.8 ± 2.7 |
| Parameters | MD ± SD |
|---|---|
| Thin endometrium (mm) | 6.7 ± 1.32 |
| SET (single embryo transfer) | 1 ± 1.18 |
| Testosterone (ng/mL) | 0.495 ± 0.62 |
| Pregnancy before PRG treatment | 0 ± 0.38 |
| Parameters | MD ± SD |
|---|---|
| Thin endometrium (mm) | 7.81 ± 0.71 |
| SET (single embryo transfer) | 2 ± 1.08 |
| Testosterone (ng/ML) | 0.797 ± 0.92 |
| Pregnancy after PRG treatment | 0.5 ± 0.12 |
| 1–7 mm | 7.1–12 mm | p Value | |||
|---|---|---|---|---|---|
| Control Group | Study Group (the Same Group after PRP Treatment) | ||||
| Age | n | % | n | % | |
| ≤35 | 32 | 62.74% | 31 | 60.78% | 0.0001 |
| >35 | 19 | 37.26% | 17 | 33.33% | 0.029 |
| Parameters | MD ± SD | p Value |
|---|---|---|
| Thin endometrium (mm) | 7.25 ± 1.015 | 0.0001 |
| SET (single embryo transfer) | 1.5 ± 1.13 | 0.0019 |
| Pregnancy after PRG treatment | 0.25 ± 0.2 | 0.0004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huniadi, A.; Zaha, I.A.; Naghi, P.; Stefan, L.; Sachelarie, L.; Bodog, A.; Szuhai-Bimbo, E.; Macovei, C.; Sandor, M. Autologous Platelet-Rich Plasma (PRP) Efficacy on Endometrial Thickness and Infertility: A Single-Centre Experience from Romania. Medicina 2023, 59, 1532. https://doi.org/10.3390/medicina59091532
Huniadi A, Zaha IA, Naghi P, Stefan L, Sachelarie L, Bodog A, Szuhai-Bimbo E, Macovei C, Sandor M. Autologous Platelet-Rich Plasma (PRP) Efficacy on Endometrial Thickness and Infertility: A Single-Centre Experience from Romania. Medicina. 2023; 59(9):1532. https://doi.org/10.3390/medicina59091532
Chicago/Turabian StyleHuniadi, Anca, Ioana Alexandra Zaha, Petronela Naghi, Liana Stefan, Liliana Sachelarie, Alin Bodog, Erika Szuhai-Bimbo, Codruta Macovei, and Mircea Sandor. 2023. "Autologous Platelet-Rich Plasma (PRP) Efficacy on Endometrial Thickness and Infertility: A Single-Centre Experience from Romania" Medicina 59, no. 9: 1532. https://doi.org/10.3390/medicina59091532