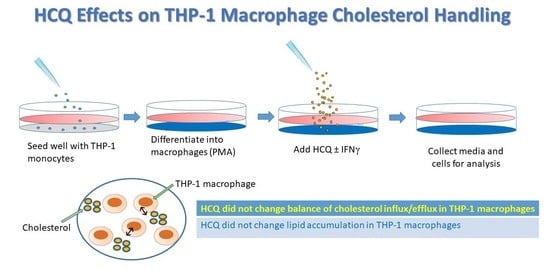
Hydroxychloroquine Effects on THP-1 Macrophage Cholesterol Handling: Cell Culture Studies Corresponding to the TARGET Cardiovascular Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Experimental Conditions
2.2. Cell Culture: Trypan Blue Viability Staining
2.3. RNA Isolation and QRT-PCR
2.4. Foam Cell Formation Assay
2.5. OxLDL Uptake
2.6. Cholesterol Efflux Analysis
2.7. pH Assay
2.8. Protein Isolation and Western Blotting
2.9. Statistical Analysis of Experimental Data
3. Results
3.1. Gene Expression–Efflux Genes
3.2. Gene Expression–Scavenger Receptors
3.3. Protein Expression–Efflux Proteins
3.4. Protein Expression–Scavenger Receptors
3.5. Viability
3.6. Cholesterol Efflux
3.7. Lipid Uptake, Staining and Foam Cells
3.8. pH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibofsky, A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: A synopsis. Am. J. Manag. Care 2014, 20, S128–S135. [Google Scholar] [PubMed]
- Scott, D.L.; Wolfe, F.; Huizinga, T. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: A systematic analysis of the global burden of disease study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef]
- Tedeschi, S.K.; Bermas, B.; Costenbader, K.H. Sexual disparities in the incidence and course of SLE and RA. Clin. Immunol. 2013, 149, 211–218. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef]
- Semb, A.G.; Kvien, T.K.; Aastveit, A.H.; Jungner, I.; Pedersen, T.R.; Walldius, G.; Holme, I. Lipids, myocardial infarction and ischaemic stroke in patients with rheumatoid arthritis in the Apolipoprotein-related Mortality RISk (AMORIS) Study. Ann. Rheum. Dis. 2010, 69, 1996–2001. [Google Scholar] [CrossRef]
- Toms, T.E.; Symmons, D.P.; Kitas, G.D. Dyslipidaemia in rheumatoid arthritis: The role of inflammation, drugs, lifestyle and genetic factors. Curr. Vasc. Pharmacol. 2010, 8, 301–326. [Google Scholar] [CrossRef]
- Holmqvist, M.E.; Wedrén, S.; Jacobsson, L.T.; Klareskog, L.; Nyberg, F.; Rantapää-Dahlqvist, S.; Alfredsson, L.; Askling, J. Rapid increase in myocardial infarction risk following diagnosis of rheumatoid arthritis amongst patients diagnosed between 1995 and 2006. J. Intern. Med. 2010, 268, 578–585. [Google Scholar] [CrossRef]
- Koren Krajnc, M.; Hojs, R.; Holc, I.; Knez, Ž.; Pahor, A. Accelerated atherosclerosis in premenopausal women with rheumatoid arthritis—15-year follow-up. Bosn. J. Basic Med. Sci. 2021, 21, 477–483. [Google Scholar] [CrossRef]
- Lindhardsen, J.; Ahlehoff, O.; Gislason, G.H.; Madsen, O.R.; Olesen, J.B.; Torp-Pedersen, C.; Hansen, P.R. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: A Danish nationwide cohort study. Ann. Rheum. Dis. 2011, 70, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Swaby, O.R.; Kuriya, B. Awareness and perceived risk of cardiovascular disease among individuals living with rheumatoid arthritis is low: Results of a systematic literature review. Arthritis Res. Ther. 2019, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Rist, P.M.; Liao, K.P.; Tawakol, A.; Fayad, Z.A.; Mani, V.; Paynter, N.P.; Ridker, P.M.; Glynn, R.J.; Lu, F.; et al. Testing the Effects of Disease-Modifying Antirheumatic Drugs on Vascular Inflammation in Rheumatoid Arthritis: Rationale and Design of the TARGET Trial. ACR Open Rheumatol. 2021, 3, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward rheumatoid arthritis therapy; the old and the new. J. Cell. Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D.; Parke, A.L.; Clifford-Rashotte, M.; Kean, W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015, 23, 231–269. [Google Scholar] [CrossRef]
- Wu, C.L.; Chang, C.C.; Kor, C.T.; Yang, T.H.; Chiu, P.F.; Tarng, D.C.; Hsu, C.C. Hydroxychloroquine Use and Risk of CKD in Patients with Rheumatoid Arthritis. Clin. J. Am. Soc. Nephrol. 2018, 13, 702–709. [Google Scholar] [CrossRef]
- van den Borne, B.E.; Dijkmans, B.A.; de Rooij, H.H.; le Cessie, S.; Verweij, C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997, 24, 55–60. [Google Scholar] [PubMed]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Solomon, D.H.; Garg, R.; Lu, B.; Todd, D.J.; Mercer, E.; Norton, T.; Massarotti, E. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: A randomized, blinded crossover trial. Arthritis Care Res. (Hoboken) 2014, 66, 1246–1251. [Google Scholar] [CrossRef]
- Tamai, R.; Sugawara, S.; Takeuchi, O.; Akira, S.; Takada, H. Synergistic effects of lipopolysaccharide and interferon-gamma in inducing interleukin-8 production in human monocytic THP-1 cells is accompanied by up-regulation of CD14, Toll-like receptor 4, MD-2 and MyD88 expression. J. Endotoxin Res. 2003, 9, 145–153. [Google Scholar] [CrossRef]
- Sumiya, Y.; Ishikawa, M.; Inoue, T.; Inui, T.; Kuchiike, D.; Kubo, K.; Uto, Y.; Nishikata, T. Macrophage Activation Mechanisms in Human Monocytic Cell Line-derived Macrophages. Anticancer Res. 2015, 35, 4447–4451. [Google Scholar]
- Dallagi, A.; Girouard, J.; Hamelin-Morrissette, J.; Dadzie, R.; Laurent, L.; Vaillancourt, C.; Lafond, J.; Carrier, C.; Reyes-Moreno, C. The activating effect of IFN-γ on monocytes/macrophages is regulated by the LIF-trophoblast-IL-10 axis via Stat1 inhibition and Stat3 activation. Cell. Mol. Immunol. 2015, 12, 326–341. [Google Scholar] [CrossRef]
- Chang, C.K.; Cheng, W.C.; Ma, W.L.; Chen, P.K.; Chen, C.H.; Shen, P.C.; Chen, C.C.; Chang, S.H.; Lai, Y.H.; Chen, D.Y. The Potential Role of Electronegative High-Density Lipoprotein H5 Subfraction in RA-Related Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 11419. [Google Scholar] [CrossRef]
- Qin, Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 2012, 221, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Arain, H.A.; Kasselman, L.J.; Renna, H.A.; Zhen, J.; Voloshyna, I.; DeLeon, J.; Carsons, S.E.; Petri, M. Human Lupus Plasma Pro-Atherogenic Effects on Cultured Macrophages Are Not Mitigated by Statin Therapy: A Mechanistic LAPS Substudy. Medicina 2019, 55, 514. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vasc. Pharmacol. 2019, 112, 54–71. [Google Scholar] [CrossRef]
- Kato, M. New insights into IFN-γ in rheumatoid arthritis: Role in the era of JAK inhibitors. Immunol. Med. 2020, 43, 72–78. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.E.; Ramji, D.P. Interferon gamma: A master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009, 20, 125–135. [Google Scholar] [CrossRef]
- Voloshyna, I.; Littlefield, M.J.; Reiss, A.B. Atherosclerosis and Interferon-γ: New Insights and Therapeutic Targets. Trends Cardiovasc. Med. 2014, 24, 45–51. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Ding, C.; Zhang, W.; Guan, H.; Li, C.; Lin, X.; Zhang, Y.; Huang, C.; Zhang, L.; et al. LncRNA H19 regulates macrophage polarization and promotes Freund’s complete adjuvant-induced arthritis by upregulating KDM6A. Int. Immunopharmacol. 2021, 93, 107402. [Google Scholar] [CrossRef]
- Narayan, N.; Mandhair, H.; Smyth, E.; Dakin, S.G.; Kiriakidis, S.; Wells, L.; Owen, D.; Sabokbar, A.; Taylor, P. The macrophage marker translocator protein (TSPO) is down-regulated on pro-inflammatory ‘M1’ human macrophages. PLoS ONE 2017, 12, e0185767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, A.B.; Carsons, S.E.; Anwar, K.; Rao, S.; Edelman, S.D.; Zhang, H.; Fernandez, P.; Cronstein, B.N.; Chan, E.S. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 2008, 58, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Sulli, A.; Craviotto, C.; Felli, L.; Pizzorni, C.; Seriolo, B.; Villaggio, B. Antiproliferative-antiinflammatory effects of methotrexate and sex hormones on cultured differentiating myeloid monocytic cells (THP-1). Ann. N. Y. Acad. Sci. 2002, 966, 232–237. [Google Scholar] [CrossRef]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef]
- Voloshyna, I.; Modayil, S.; Littlefield, M.J.; Belilos, E.; Belostocki, K.; Bonetti, L.; Rosenblum, G.; Carsons, S.E.; Reiss, A.B. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp. Biol. Med. (Maywood) 2013, 238, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Taylor, J.M.; Graham, A. Mitochondrial (dys)function and regulation of macrophage cholesterol efflux. Clin. Sci. (Lond.) 2013, 124, 509–515. [Google Scholar] [CrossRef]
- Chen, D.Y.; Chih, H.M.; Lan, J.L.; Chang, H.Y.; Chen, W.W.; Chiang, E.P. Blood lipid profiles and peripheral blood mononuclear cell cholesterol metabolism gene expression in patients with and without methotrexate treatment. BMC Med. 2011, 9, 4. [Google Scholar] [CrossRef]
- Graham, A.; Allen, A.M. Mitochondrial function and regulation of macrophage sterol metabolism and inflammatory responses. World J. Cardiol. 2015, 7, 277–286. [Google Scholar] [CrossRef]
- Mäkinen, P.I.; Lappalainen, J.P.; Heinonen, S.E.; Leppänen, P.; Lähteenvuo, M.T.; Aarnio, J.V.; Heikkilä, J.; Turunen, M.P.; Ylä-Herttuala, S. Silencing of either SR-A or CD36 reduces atherosclerosis in hyperlipidaemic mice and reveals reciprocal upregulation of these receptors. Cardiovasc. Res. 2010, 88, 530–538. [Google Scholar] [CrossRef]
- Kunjathoor, V.V.; Febbraio, M.; Podrez, E.A.; Moore, K.J.; Andersson, L.; Koehn, S.; Rhee, J.S.; Silverstein, R.; Hoff, H.F.; Freeman, M.W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002, 277, 49982–49988. [Google Scholar] [CrossRef]
- Bathon, J.M. Is the Gap in Incidence of Cardiovascular Events in Rheumatoid Arthritis Really Closing? J. Rheumatol. 2021, 48, 1351–1353. [Google Scholar] [CrossRef]
- Guevara, M.; Ng, B. Positive effect of hydroxychloroquine on lipid profiles of patients with rheumatoid arthritis: A Veterans Affair cohort. Eur. J. Rheumatol. 2020, 5, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, J.F.; Del Rincon, I.; Molina, E.; Battafarano, D.F.; Escalante, A. Use of Hydroxychloroquine Is Associated with Improved Lipid Profile in Rheumatoid Arthritis Patients. J. Clin. Rheumatol. 2017, 23, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Zhang, S.; Silverman, G.; Li, M.; Cai, J.; Niu, H. Protective effect of hydroxychloroquine on rheumatoid arthritis-associated atherosclerosis. Animal Model. Exp. Med. 2019, 2, 98–106. [Google Scholar] [CrossRef]
- Hartman, O.; Kovanen, P.T.; Lehtonen, J.; Eklund, K.K.; Sinisalo, J. Hydroxychloroquine for the prevention of recurrent cardiovascular events in myocardial infarction patients: Rationale and design of the OXI trial. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 92–97. [Google Scholar] [CrossRef]
- Ulander, L.; Tolppanen, H.; Hartman, O.; Rissanen, T.T.; Paakkanen, R.; Kuusisto, J.; Anttonen, O.; Nieminen, T.; Yrjölä, J.; Ryysy, R.; et al. Hydroxychloroquine reduces interleukin-6 levels after myocardial infarction: The randomized, double-blind, placebo-controlled OXI pilot trial. Int. J. Cardiol. 2021, 337, 21–27. [Google Scholar] [CrossRef]
- Lang, M.G.; Vinagre, C.G.; Bonfa, E.; Freitas, F.R.; Pasoto, S.G.; Brito, T.S.; Seguro, L.P.; Maranhão, R.C.; Borba, E.F. Hydroxychloroquine increased cholesterol transfer to high-density lipoprotein in systemic lupus erythematosus: A possible mechanism for the reversal of atherosclerosis in the disease. Lupus 2022, 31, 659–665. [Google Scholar] [CrossRef]







| Gene | Sequence |
|---|---|
| ABCA1 | Forward: 5′-GAAGTACATCAGAACATGGGC-3′ Reverse: 5′-GATCAAAGCCATGGCTGTAG-3′ |
| ABCG1 | Forward: 5′-CAGGAAGATTAGACACTGTGG-3′ Reverse: 5′-GAAAGGGGAATGGAGAGAAG-3′ |
| CYP27A1 | Forward: 5′-AAGCGATACCTGGATGGTTG-3′ Reverse: 5′-TGTTGGATGTCGTGTCCACT-3′ |
| CD36 | Forward: 5′-GAGAACTGTTATGGGGCTAT-3′ Reverse: 5′-TTCAACTGGAGAG-GCAAAGG-3′ |
| SRA1 | Forward: 5′-CTCGTGTTTGCAGTTCTCA-3′ Reverse: 5′-CCATGTTGCTCATGTGTTCC-3′ |
| GAPDH | Forward: 5′-ACCATCATCCCTGCCTCTAC-3′ Reverse: 5′-CCTGTTGCTGTAGCCAAAT-3′ |
| Name | Description |
|---|---|
| ABCA1 | A full-size ABC transporter that mediates active efflux of cholesterol from macrophages and other nonhepatic cells to lipid-free apolipoprotein A-I (prevents foam cell formation). Essential for the generation of high density lipoprotein (HDL). |
| ABCG1 | Mediates the removal of lipid molecules, including cholesterol and phospholipids, from macrophages and their transport across cellular and intracellular membranes to HDL particles. |
| Cholesterol 27-Hydroxylase (CYP27A1) | A mitochondrial enzyme that catalyzes the hydroxylation of cholesterol to more polar oxysterol metabolites that exit cells through lipid membranes orders of magnitude faster than cholesterol. It also regulates cholesterol biosynthesis via its major metabolite, 27-hydroxycholesterol, which potently inhibits cholesterol synthesis. |
| CD36 | A member of the class B scavenger receptor family crucial for macrophage uptake of modified LDL, primarily oxidized LDL |
| SRA1 | Expressed mainly by mature macrophages. Binds to oxidized LDL and other modified forms of LDL and mediates their internalization. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Konig, J.; Kasselman, L.J.; Renna, H.A.; De Leon, J.; Carsons, S.E.; Reiss, A.B. Hydroxychloroquine Effects on THP-1 Macrophage Cholesterol Handling: Cell Culture Studies Corresponding to the TARGET Cardiovascular Trial. Medicina 2022, 58, 1287. https://doi.org/10.3390/medicina58091287
Ahmed S, Konig J, Kasselman LJ, Renna HA, De Leon J, Carsons SE, Reiss AB. Hydroxychloroquine Effects on THP-1 Macrophage Cholesterol Handling: Cell Culture Studies Corresponding to the TARGET Cardiovascular Trial. Medicina. 2022; 58(9):1287. https://doi.org/10.3390/medicina58091287
Chicago/Turabian StyleAhmed, Saba, Justin Konig, Lora J. Kasselman, Heather A. Renna, Joshua De Leon, Steven E. Carsons, and Allison B. Reiss. 2022. "Hydroxychloroquine Effects on THP-1 Macrophage Cholesterol Handling: Cell Culture Studies Corresponding to the TARGET Cardiovascular Trial" Medicina 58, no. 9: 1287. https://doi.org/10.3390/medicina58091287