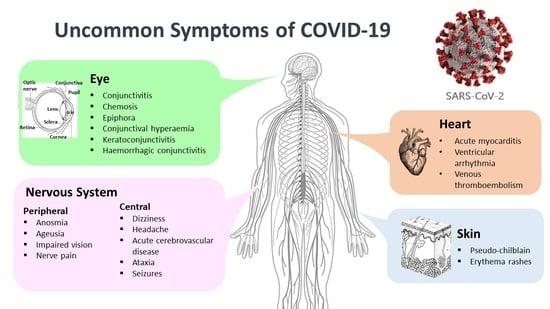
Focused Review: Potential Rare and Atypical Symptoms as Indicator for Targeted COVID-19 Screening
Abstract
:1. Introduction
2. Research Methodology
3. Neurological Symptoms
4. Cutaneous Symptoms
5. Ocular Symptoms
6. Cardiovascular Symptoms
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goh, H.P.; Mahari, W.I.; Ahad, N.I.; Chaw, L.L.; Kifli, N.; Goh, B.-H.; Yeoh, S.F.; Goh, K.W.; Ming, L.C. Risk factors affecting COVID-19 case fatality rate: A quantitative analysis of top 50 affected countries. Prog. Microbes Mol. Biol. 2020, 3, a0000171. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 9 January 2021).
- Gencer, S.; Lacy, M.; Atzler, D.; van der Vorst, E.P.C.; Doring, Y.; Weber, C. Immunoinflammatory, Thrombohaemostatic, and Cardiovascular Mechanisms in COVID-19. Thromb. Haemost. 2020, 120, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Yeoh, S.F.; Long, C.M. COVID-19: Critical Role of Angiotensin 1–7 in ACE2 Modulation. Ann. Acad. Med. 2020, 49, 398–400. [Google Scholar]
- National Health Commission & State Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia, 7th ed.; Trial Version; Wei, P.-F., Ed.; National Health Commission of the People’s Republic of China: Beijing, China, 2020; p. 17.
- Kalra, R.S.; Tomar, D.; Meena, A.S.; Kandimalla, R. SARS-CoV-2, ACE2, and Hydroxychloroquine: Cardiovascular Complications, Therapeutics, and Clinical Readouts in the Current Settings. Pathogens 2020, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Kowalewski, M.; Żekanowska, E. Coronavirus disease 2019 (COVID–19): A short review on hematological manifestations. Pathogens 2020, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—3 August 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. The COVID-19 Candidate Vaccine Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 9 January 2021).
- U.S. Food & Drug Administration. COVID-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 9 January 2021).
- European Medicines Agency. EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu (accessed on 9 January 2021).
- Ng, S.L.; Soon, T.N.; Yap, W.H.; Liew, K.B.; Lim, Y.C.; Ming, L.C. Convalescent plasma: A potential therapeutic option for COVID-19 patients. Asian Pac. J. Trop. Med. 2020, 13, 477. [Google Scholar]
- Favas, T.T.; Dev, P.; Chaurasia, R.N.; Chakravarty, K.; Mishra, R.; Joshi, D.; Mishra, V.N.; Kumar, A.; Singh, V.K.; Pandey, M.; et al. Neurological manifestations of COVID-19: A systematic review and meta-analysis of proportions. Neurol. Sci. 2020, 41, 3437–3470. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, Y.; Li, M.; Chuang, H.; Ye, Y.; Zhao, H.; Wang, H. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Neurol. 2020, 267, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Candan, S.A.; Abba, M.A.; Bello, A.H.; Alshehri, M.A.; Afamefuna Victor, E.; Umar, N.A.; Kundakci, B. Neurological and Musculoskeletal Features of COVID-19: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Pinzon, R.T.; Wijaya, V.O.; Buana, R.B.; Al Jody, A.; Nunsio, P.N. Neurologic Characteristics in Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 565. [Google Scholar] [CrossRef]
- Ghannam, M.; Alshaer, Q.; Al-Chalabi, M.; Zakarna, L.; Robertson, J.; Manousakis, G. Neurological involvement of coronavirus disease 2019: A systematic review. J. Neurol. 2020. [Google Scholar] [CrossRef]
- Kelvin Oliveira, R.; Virgínia Vinha, Z.; Flávia Diniz, V.; Luciana Moreira, L. COVID-19 and Cutaneous Disorders: What’s Being Reported? A Meta-Analysis from Observational Studies and Case Reports. J. Port. Soc. Dermatol. Venereol. 2020, 78. [Google Scholar] [CrossRef]
- Conforti, C.; Dianzani, C.; Agozzino, M.; Giuffrida, R.; Marangi, G.F.; di Meo, N.; Morariu, S.-H.; Persichetti, P.; Segreto, F.; Zalaudek, I.; et al. Cutaneous Manifestations in Confirmed COVID-19 Patients: A Systematic Review. Biology 2020, 9, 449. [Google Scholar] [CrossRef]
- Lee, D.S.; Mirmirani, P.; McCleskey, P.E.; Mehrpouya, M.; Gorouhi, F. Cutaneous manifestations of COVID-19: A systematic review and analysis of individual patient-level data. Dermatol. Online J. 2020, 26, 13030/qt7s34p8rw. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Agarwal, A.; Jaiswal, N.; Dahiya, N.; Ahuja, A.; Mahajan, S.; Tong, L.; Duggal, M.; Singh, M.; Agrawal, R.; et al. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241661. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Kline, B.; Han, Y.; Ying, G.-s.; Wang, N.L. Current Evidence of 2019 Novel Coronavirus Disease (COVID-19) Ocular Transmission: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 7605453. [Google Scholar] [CrossRef] [PubMed]
- Vakhshoori, M.; Heidarpour, M.; Shafie, D.; Taheri, M.; Rezaei, N.; Sarrafzadegan, N. Acute Cardiac Injury in COVID-19: A Systematic Review and Meta-analysis. Arch. Iran. Med. 2020, 23, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Guo, J. Clinical Manifestations of Myocardial Injury Associated with the 2019 Novel Coronavirus Disease in Chinese Population: A Meta-analysis. Int. J. Infect. Dis. Ther. 2020, 5, 136–144. [Google Scholar] [CrossRef]
- Potere, N.; Valeriani, E.; Candeloro, M.; Tana, M.; Porreca, E.; Abbate, A.; Spoto, S.; Rutjes, A.W.S.; Di Nisio, M. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: A systematic review and meta-analysis. Crit. Care 2020, 24, 389. [Google Scholar] [CrossRef] [PubMed]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 2020, 29. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus: Symptoms. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_3 (accessed on 23 January 2021).
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi-Pooya, A.A.; Simani, L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020, 413, 116832. [Google Scholar] [CrossRef]
- Harzallah, I.; Debliquis, A.; Drénou, B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemost. [CrossRef]
- Zhang, Y.; Cao, W.; Xiao, M.; Li, Y.J.; Yang, Y.; Zhao, J.; Zhou, X.; Jiang, W.; Zhao, Y.Q.; Zhang, S.Y.; et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Chin. J. Hematol. 2020, 41, E006. [Google Scholar] [CrossRef]
- Wanleenuwat, P.; Iwanowski, P.; Kozubski, W. Antiganglioside antibodies in neurological diseases. J. Neurol. Sci. 2020, 408, 116576. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Ostrander, B.T.; DeConde, A.S. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 821–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.u.; Kim, M.J.; Ra, S.H.; Lee, J.; Bae, S.; Jung, J.; Kim, S.H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin. Microbiol. Infect. 2020, 26, 948.e1–948.e3. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G.; et al. Self-reported Olfactory and Taste Disorders in Patients with Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Arch. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Luers, J.C.; Rokohl, A.C.; Loreck, N.; Wawer Matos, P.A.; Augustin, M.; Dewald, F.; Klein, F.; Lehmann, C.; Heindl, L.M. Olfactory and Gustatory Dysfunction in Coronavirus Disease 19 (COVID-19). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.-H.; Sridhar, S.; Zhang, A.J.; Chan, K.-H.; Li, H.-L.; Wong, F.K.-C.; Ng, M.-Y.; Tsang, R.K.-Y.; Lee, A.C.-Y.; Fan, Z.; et al. Olfactory Dysfunction in Coronavirus Disease 2019 Patients: Observational Cohort Study and Systematic Review. Open Forum Infect. Dis. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Gane, S.B.; Kelly, C.; Hopkins, C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology 2020, 58, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shan, K.S.; Abdollahi, S.; Nace, T. Anosmia and Ageusia as the Only Indicators of Coronavirus Disease 2019 (COVID-19). Cureus 2020, 12, e7918. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Moein, S.T.; Hashemian, S.M.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell dysfunction: A biomarker for COVID-19. Int Forum Allergy Rhinol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, T.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.Y.; Lepiller, Q.; Gendrin, V.; Zayet, S. Features of anosmia in COVID-19. Med. Mal. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.-W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35. [Google Scholar] [CrossRef]
- Lu, L.; Xiong, W.; Liu, D.; Liu, J.; Yang, D.; Li, N.; Mu, J.; Guo, J.; Li, W.; Wang, G.; et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia 2020, 61, e49–e53. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Jiang, D.; Huang, J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav. Immun. 2020, 87, 149. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Filatov, A.; Sharma, P.; Hindi, F.; Espinosa, P.S. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 2020, 12, e7352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzano, A.V.; Cassano, N.; Genovese, G.; Moltrasio, C.; Vena, G.A. Cutaneous manifestations in patients with COVID-19: A preliminary review of an emerging issue. Br. J. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Daneshgaran, G.; Dubin, D.P.; Gould, D.J. Cutaneous Manifestations of COVID-19: A Systematic Review. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Matar, S.; Oulès, B.; Sohier, P.; Chosidow, O.; Beylot-Barry, M.; Dupin, N.; Aractingi, S. Cutaneous manifestations in SARS-CoV-2 infection (COVID-19): A French experience and a systematic review of the literature. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Fang, X.; Pang, Z.; Zhang, B.; Liu, H.; Zhang, F. COVID-19 and cutaneous manifestations: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Nieto, D.; Jimenez-Cauhe, J.; Suarez-Valle, A.; Moreno-Arrones, O.M.; Saceda-Corralo, D.; Arana-Raja, A.; Ortega-Quijano, D. Characterization of acute acral skin lesions in nonhospitalized patients: A case series of 132 patients during the COVID-19 outbreak. J. Am. Acad. Dermatol. 2020, 83, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.; Kaur, H.; Kaur, H.; Bhattacharyya, J.; Prajapat, M.; Shekhar, N.; Avti, P.; Kumar, S.; Medhi Medhi, B.; Das, D.; et al. Ocular Manifestations and Tear or Conjunctival Swab PCR Positivity for 2019-nCoV in Patients with COVID-19: A Systematic Review and Meta-Analysis. SSRN 2020. [Google Scholar] [CrossRef]
- Loffredo, L.; Pacella, F.; Pacella, E.; Tiscione, G.; Oliva, A.; Violi, F. Conjunctivitis and COVID-19: A meta-analysis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Casalino, G.; Monaco, G.; Di Sarro, P.P.; David, A.; Scialdone, A. Coronavirus disease 2019 presenting with conjunctivitis as the first symptom. Eye 2020, 34, 1235–1236. [Google Scholar] [CrossRef] [PubMed]
- Khavandi, S.; Tabibzadeh, E.; Naderan, M.; Shoar, S. Corona virus disease-19 (COVID-19) presenting as conjunctivitis: Atypically high-risk during a pandemic. Cont. Lens Anterior Eye 2020, 43, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Cheema, M.; Aghazadeh, H.; Nazarali, S.; Ting, A.; Hodges, J.; McFarlane, A.; Kanji, J.N.; Zelyas, N.; Damji, K.F.; Solarte, C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Daruich, A.; Martin, D.; Bremond-Gignac, D. Ocular manifestation as first sign of Coronavirus Disease 2019 (COVID-19): Interest of telemedicine during the pandemic context. J. Fr. Ophtalmol. 2020, 43, 389–391. [Google Scholar] [CrossRef]
- Navel, V.; Chiambaretta, F.; Dutheil, F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. Am. J. Ophthalmol. Case Rep. 2020, 19, 100735. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578. [Google Scholar] [CrossRef]
- La Distia Nora, R.; Putera, I.; Khalisha, D.F.; Septiana, I.; Ridwan, A.S.; Sitompul, R. Are eyes the windows to COVID-19? Systematic review and meta-analysis. BMJ Open Ophthalmol. 2020, 5, e000563. [Google Scholar] [CrossRef]
- Karimi, S.; Arabi, A.; Shahraki, T.; Safi, S. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with Coronavirus disease 2019. Eye 2020, 34, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, F.; Gallo Afflitto, G.; Mancino, R.; Li, J.-P.O.; Cesareo, M.; Giannini, C.; Nucci, C. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: A systematic review. Eye 2020, 34, 1206–1211. [Google Scholar] [CrossRef]
- Khan, I.H.; Zahra, S.A.; Zaim, S.; Harky, A. At the heart of COVID-19. J. Card. Surg. 2020, 35, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Myocardial injury in patients with COVID-19. Nat. Rev. Cardiol. 2020, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Petersen-Uribe, Á.; Avdiu, A.; Witzel, K.; Jaeger, P.; Zdanyte, M.; Heinzmann, D.; Tavlaki, E.; Müller, K.; Gawaz, M.P. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin. Res. Cardiol. 2020. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
| Symptoms | Comments | Prevalence | Citations |
|---|---|---|---|
| Anosmia | Prevalent in younger patients, females, occurs in early stages of COVID-19 | 35.8% | Favas et al. [13] |
| 35.7–85.6% | Wang et al. [14] | ||
| 35% | Abdullahi et al. [15] | ||
| Ageusia | 38.5% | Favas et al. [13] | |
| 33.3–88.8% | Wang et al. [14] | ||
| 33% | Abdullahi et al. [15] | ||
| Headache | Tend to be found in young patients, occurs in early stages of COVID-19 | 14.7% | Favas et al. [13] |
| 12% | Abdullahi et al. [15] | ||
| 10.9% | Pinzon et al. [16] | ||
| Acute cerebrovascular disease | Onset: 9–10 days; Prevalent in older patients; associated with severe form of COVID-19 | 2.3% | Favas et al. [13] |
| 3% | Abdullahi et al. [15] | ||
| 4.4% | Pinzon et al. [16] | ||
| Ischaemic stroke | 2.1% | Favas et al. [13] | |
| Haemorrhagic stroke | 0.4% | Favas et al. [13] | |
| Guillain–Barré syndrome | Onset: 3–24 days | 4 case reports | Wang et al. [14] |
| 73.9% (n = 17) | Ghannam et al. [17] | ||
| Maculopapular rash | Onset: 8 days, commonly found in females | 37.5% | Rocha et al. [18] |
| 38% | Conforti et al. [19] | ||
| Chilblain-like | In younger patients with median age: 20; median onset: 5 days; associated with mild disease | 10% | Rocha et al. [18] |
| 12.8% | Conforti et al. [19] | ||
| 18% | Lee et al. [20] | ||
| Acro-ischemia | Associated with severe disease; Onset: 19 days | 9% | Lee et al. [20] |
| Kawasaki disease-like presentation | Onset: 3.5 days; commonly found in males | 6.9% | Lee et al. [20] |
| 0.8% | Conforti et al. [19] | ||
| Polymorphic patterns | Rare symptoms; requires further investigation | 1.4% | Conforti et al. [19] |
| Generalized pruritus | 1.2% | Conforti et al. [19] | |
| Atypical erythema nodosum | 0.5% | Conforti et al. [19] | |
| Atypical Sweet syndrome | 0.2% | Conforti et al. [19] | |
| Ocular redness | Can be presented as first symptom or developed during disease progression | 10.9% | Aggarwal et al. [21] |
| Conjunctivitis | 7% | Aggarwal et al. [21] | |
| 8.3% | Cao et al. [22] | ||
| Conjunctival chemosis | 4.4% | Aggarwal et al. [21] | |
| Acute myocardial injury | Associated with severe form of COVID-19; Predominant in males, median age: 56 | 15% | Vakhshoori et al. [23] |
| 18% | Gong and Guo [24] | ||
| 15% | Potore et al. [25] | ||
| Venous Thromboembolism | More common in COVID-19 patients in intensive care unit, increased odds of mortality | 15%21% | Potore et al. [25] Malas et al. [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.L.; Ong, Y.S.; Khaw, K.Y.; Teh, S.P.; Tan, C.S.; Ming, L.C.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Focused Review: Potential Rare and Atypical Symptoms as Indicator for Targeted COVID-19 Screening. Medicina 2021, 57, 189. https://doi.org/10.3390/medicina57020189
Ng SL, Ong YS, Khaw KY, Teh SP, Tan CS, Ming LC, Chan K-G, Lee L-H, Goh B-H. Focused Review: Potential Rare and Atypical Symptoms as Indicator for Targeted COVID-19 Screening. Medicina. 2021; 57(2):189. https://doi.org/10.3390/medicina57020189
Chicago/Turabian StyleNg, Swee Li, Yong Sze Ong, Kooi Yeong Khaw, Siew Phooi Teh, Ching Siang Tan, Long Chiau Ming, Kok-Gan Chan, Learn-Han Lee, and Bey-Hing Goh. 2021. "Focused Review: Potential Rare and Atypical Symptoms as Indicator for Targeted COVID-19 Screening" Medicina 57, no. 2: 189. https://doi.org/10.3390/medicina57020189