Rapid Effect of Benralizumab for Hypereosinophilia in a Case of Severe Asthma with Eosinophilic Chronic Rhinosinusitis
Abstract
:1. Introduction
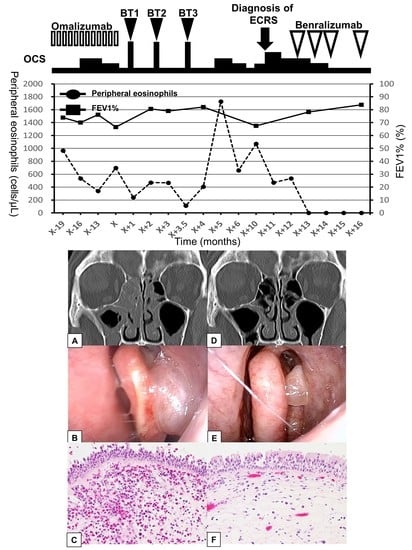
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Asthma. 2018 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/ (accessed on 6 April 2019).
- Laviolette, M.; Gossage, D.L.; Gauvreau, G.; Leigh, R.; Olivenstein, R.; Katial, R.; Busse, W.W.; Wenzel, S.; Wu, Y.; Datta, V.; et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J. Allergy Clin. Immunol. 2013, 132, 1086–1096.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Tokunaga, T.; Sakashita, M.; Haruna, T.; Asaka, D.; Takeno, S.; Ikeda, H.; Nakayama, T.; Seki, N.; Ito, S.; Murata, J.; et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: The JESREC Study. Allergy 2015, 70, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Hirano, T.; Akamatsu, K.; Koarai, A.; Sugiura, H.; Minakata, Y.; Ichinose, M. Exhaled nitric oxide cutoff values for asthma diagnosis according to rhinitis and smoking status in Japanese subjects. Allergol. Int. 2011, 60, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Hisada, T.; Kamide, Y.; Aoki, H.; Seki, K.; Honjo, C.; Sakai, H.; Kadowaki, M.; Umeda, Y.; Morikawa, M.; et al. The effects of concomitant GERD, dyspepsia, and rhinosinusitis on asthma symptoms and FeNO in asthmatic patients taking controller medications. J. Asthma Allergy 2014, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kambara, R.; Minami, T.; Akazawa, H.; Tsuji, F.; Sasaki, T.; Inohara, H.; Horii, A. Lower Airway Inflammation in Eosinophilic Chronic Rhinosinusitis as Determined by Exhaled Nitric Oxide. Int. Arch. Allergy Immunol 2017, 173, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Asako, M.; Ooka, H.; Kanda, A.; Tomoda, K.; Yasuba, H. Residual exhaled nitric oxide elevation in asthmatics is associated with eosinophilic chronic rhinosinusitis. J. Asthma 2015, 52, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Guyatt, G.H.; Epstein, R.S.; Ferrie, P.J.; Jaeschke, R.; Hiller, T.K. Evaluation of impairment of health related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax 1992, 47, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Bleecker, E.R.; FitzGerald, J.M.; Ferguson, G.T.; Barker, P.; Sproule, S.; Olsson, R.F.; Martin, U.J.; Goldman, M.; BORA Study Investigators. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir. Med. 2019, 7, 46–59. [Google Scholar] [CrossRef]
- Cox, G.; Thomson, N.C.; Rubin, A.S.; Niven, R.M.; Corris, P.A.; Siersted, H.C.; Olivenstein, R.; Pavord, I.D.; McCormack, D.; Chaudhuri, R.; et al. Asthma control during the year after bronchial thermoplasty. N. Engl. J. Med. 2007, 356, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Laviolette, M.; Rubin, A.S.; Fiterman, J.; Lapa e Silva, J.R.; Shah, P.L.; Fiss, E.; Olivenstein, R.; Thomson, N.C.; Niven, R.M.; et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J. Allergy Clin. Immunol. 2013, 132, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Pretolani, M.; Bergqvist, A.; Thabut, G.; Dombret, M.C.; Knapp, D.; Hamidi, F.; Alavoine, L.; Taillé, C.; Chanez, P.; Erjefält, J.S.; et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: Clinical and histopathologic correlations. J. Allergy Clin. Immunol. 2017, 139, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Gevaert, P.; Van Bruaene, N.; Cattaert, T.; Van Steen, K.; Van Zele, T.; Acke, F.; De Ruyck, N.; Blomme, K.; Sousa, A.R.; Marshall, R.P.; et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J. Allergy Clin. Immunol. 2011, 128, 989–995.e8. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Sousa, A.R.; Lund, V.J.; Scadding, G.K.; Gevaert, P.; Nasser, S.; Durham, S.R.; Cornet, M.E.; Kariyawasam, H.H.; Gilbert, J.; et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J. Allergy Clin. Immunol. 2017, 140, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Adachi, M.; Tohda, Y.; Kamei, T.; Kato, M.; Mark Fitzgerald, J.; Takanuma, M.; Kakuno, T.; Imai, N.; Wu, Y.; et al. Efficacy and safety of benralizumab in Japanese patients with severe, uncontrolled eosinophilic asthma. Allergol. Int. 2018, 67, 266–272. [Google Scholar] [CrossRef] [PubMed]


| Laboratory Data Before the Treatment of BT | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hematology | Chemistry | |||||||
| WBC | 10,400 | /μL | TP | 7.1 | g/dL | BUN | 13 | mg/dL |
| Neu | 7150 | /μL | Alb | 4.3 | g/dL | Cr | 1.02 | mg/dL |
| Eos | 690 | /μL | T-bil | 1.2 | mg/dL | Na | 141 | mEq/L |
| Bas | 110 | /μL | AST | 19 | U/L | K | 4.1 | mEq/L |
| Mon | 290 | /μL | ALT | 24 | U/L | Cl | 106 | mEq/L |
| Lym | 2110 | /μL | LDH | 268 | U/L | Ca | 9.6 | mEq/L |
| RBC | 552 | ×104/μL | ALP | 267 | U/L | BNP | 5.1 | pg/mL |
| Hb | 17.5 | g/dL | GGTP | 32 | U/L | CRP | 0.19 | mg/dL |
| Plt | 18.7 | ×104/μL | CK | 344 | U/L | Glu | 150 | mg/dL |
| UA | 6.8 | mg/dL | HbA1c | 6.1 | % | |||
| Coagulation | T-chol | 161 | mg/dL | |||||
| PT | 104 | % | HDL-C | 47 | mg/dL | Serology | ||
| APTT | 28.5 | sec | LDL-C | 91 | mg/dL | total IgE | 457.3 | IU/mL |
| D-dimer | 0.5 | μg/mL | TG | 180 | mg/dL | |||
| Serology data before the treatment of omalizumab | ||||||||
| total IgE | 267 | IU/mL | house dust mite | 8.83 | UA/mL (Class 3) | |||
| Japanese cedar | 4.68 | UA/mL (Class 3) | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurumaki, H.; Matsuyama, T.; Ezawa, K.; Koga, Y.; Yatomi, M.; Aoki-Saito, H.; Chikamatsu, K.; Hisada, T. Rapid Effect of Benralizumab for Hypereosinophilia in a Case of Severe Asthma with Eosinophilic Chronic Rhinosinusitis. Medicina 2019, 55, 336. https://doi.org/10.3390/medicina55070336
Tsurumaki H, Matsuyama T, Ezawa K, Koga Y, Yatomi M, Aoki-Saito H, Chikamatsu K, Hisada T. Rapid Effect of Benralizumab for Hypereosinophilia in a Case of Severe Asthma with Eosinophilic Chronic Rhinosinusitis. Medicina. 2019; 55(7):336. https://doi.org/10.3390/medicina55070336
Chicago/Turabian StyleTsurumaki, Hiroaki, Toshiyuki Matsuyama, Kazuma Ezawa, Yasuhiko Koga, Masakiyo Yatomi, Haruka Aoki-Saito, Kazuaki Chikamatsu, and Takeshi Hisada. 2019. "Rapid Effect of Benralizumab for Hypereosinophilia in a Case of Severe Asthma with Eosinophilic Chronic Rhinosinusitis" Medicina 55, no. 7: 336. https://doi.org/10.3390/medicina55070336