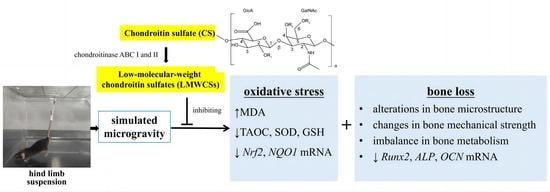
Low-Molecular-Weight Chondroitin Sulfates Alleviate Simulated Microgravity-Induced Oxidative Stress and Bone Loss in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethics Approval
2.3. Animals
2.4. Grouping and Treatment of Animals
2.5. Collection and Preparation of Tissue Samples
2.5.1. Collection of Blood Samples and Preparation of Serum
2.5.2. Collection and Treatment of the Femur and Tibia
2.5.3. Urine Collection
2.6. Determination of the Oxidative/Antioxidant Indices in the Serum and Bone Tissue
2.7. Micro-CT Analysis
2.8. Bone Mechanical Properties Test
2.9. Determination of Biochemical Indicators of Bone Metabolism
2.9.1. Determination of Alkaline Phosphatase (ALP) Activity and Osteocalcin (OCN) Levels
2.9.2. Determination of Deoxypyridinoline (DPD) and Creatinine (CRE)
2.10. qPCR Detection of mRNA Levels
2.11. Statistical Analysis
3. Results
3.1. The General Effect of LMWCSs Treatment in Mice
3.2. LMWCSs abate Oxidative Stress in HLS Mice
3.3. LMWCSs Increase the Antioxidant Capacity through Regulation of the mRNA Expression Levels of Antioxidant Enzyme-Related Genes in HLS Mice
3.4. LMWCSs Treatment Alleviates Bone Loss Induced by HLS in Mice
3.5. LMWCSs Regulate the Imbalance of Bone Metabolism in HLS Mice
3.6. LMWCSs Improve Osteoblast Activity through Regulation of the mRNA Expression Levels of Osteogenic-Related Genes in HLS Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tian, Y.; Ma, X.; Yang, C.; Su, P.; Yin, C.; Qian, A.R. The impact of oxidative stress on the bone system in response to the space special environment. Int. J. Mol. Sci. 2017, 18, 2132. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. The effects of microgravity and space radiation on cardiovascular health: From low-earth orbit and beyond. IJC Heart Vasc. 2020, 30, 100595. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; Nicole, L.; Guido, A.; Pizzol, D. Spaceflight osteoporosis: Current state and future perspective. Endocr. Regul. 2015, 49, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Traon, P.L.; Heer, M.; Narici, M.V.; Rittweger, J.; Vernikos, J. From space to earth: Advances in human physiology from 20 years of bed rest studies (1986–2006). Eur. J. Appl. Physiol. 2007, 101, 143–194. [Google Scholar] [CrossRef]
- Xin, M.; Yang, Y.; Zhang, D.; Wang, J.; Chen, S.; Zhou, D. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporos. Int. 2015, 26, 2665–2676. [Google Scholar] [CrossRef]
- Qu, L.; Chen, H.; Liu, X.; Bi, L.; Xiong, J.; Mao, Z.; Li, Y. Protective effects of flavonoids against oxidative stress induced by simulated microgravity in SH-SY5Y Cells. Neurochem. Res. 2010, 35, 1445–1454. [Google Scholar] [CrossRef]
- Niu, Y.-B.; Yang, Y.-Y.; Xiao, X.; Sun, Y.; Zhou, Y.-M.; Zhang, Y.-H.; Dong, D.; Li, C.-R.; Wu, X.-L.; Li, Y.-H.; et al. Quercetin prevents bone loss in hindlimb suspension mice via stanniocalcin 1-mediated inhibition of osteoclastogenesis. Acta Pharmacol. Sin. 2020, 41, 1476–1486. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Farran, A.; Montell, E.; Vergés, J.; Pelletier, J.P. Discrepancies in composition and biological effects of different formulations of chondroitin sulfate. Molecules 2015, 20, 4277–4289. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Campo, S.; Nastasi, G.; Traina, P.; D’ascola, A.; Rugolo, C.A.; Calatroni, A. The antioxidant activity of chondroitin-4-sulphate, in carbon tetrachloride-induced acute hepatitis in mice, involves NF-κB and caspase activation. Br. J. Pharmacol. 2010, 155, 945–956. [Google Scholar] [CrossRef]
- Rani, A.; Baruah, R.; Goyal, A. Physicochemical, antioxidant and biocompatible properties of chondroitin sulphate isolated from chicken keel bone for potential biomedical applications. Carbohydr. Polym. 2017, 159, 11–19. [Google Scholar] [CrossRef]
- Müller, A.J.; Letelier, M.E.; Galleguillos, M.A.; Molina-Berríos, A.E.; Adarmes, H.H. Comparison of the antioxidant effects of synovial fluid from equine metacarpophalangeal joints with those of hyaluronic acid and chondroitin sulfate. Am. J. Vet. Res. 2010, 71, 399–404. [Google Scholar] [CrossRef]
- Pecchi, E.; Priam, S.; Mladenovic, Z.; Gosset, M.; Saurel, A.-S.; Aguilar, L.; Berenbaum, F.; Jacques, C. A potential role of chondroitin sulfate on bone in osteoarthritis: Inhibition of prostaglandin E2 and matrix metalloproteinases synthesis in interleukin-1β- stimulated osteoblasts. Osteoarthr. Cartil. 2012, 20, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Akiyama, H.; Sato, Y.; Yoshioka, Y.; Linhardt, R.J.; Goda, Y.; Maitani, T.; Toida, T. Chondroitin sulfate intake inhibits the IgE-mediated allergic response by down-regulating Th2 responses in mice. J. Biol. Chem. 2006, 281, 19872–19880. [Google Scholar] [CrossRef]
- Han, L.-K.; Sumiyoshi, M.; Takeda, T.; Chihara, H.; Nishikiori, T.; Tsujita, T.; Kimura, Y.; Okuda, H. Inhibitory effects of chondroitin sulfate prepared from salmon nasal cartilage on fat storage in mice fed a high-fat diet. Int. J. Obesity 2000, 24, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Liu, C.; Song, C.; Li, P.; Yin, F.; Xiao, Y.; Jiang, W.; Zong, A.; Zhang, X.; et al. Protective effects of low molecular weight chondroitin sulfate on amyloid beta (Aβ)-induced damage in vitro and in vivo. Neuroscience 2015, 305, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Honvo, G.; Bruyère, O.; Geerin, A.; Veronese, N.; Reginster, J.Y. Efficacy of chondroitin sulfate in patients with knee osteoarthritis: A comprehensive meta-analysis exploring inconsistencies in randomized, placebo-controlled trials. Adv. Ther. 2019, 36, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Dudler, J.; Blicharski, T.; Pavelka, K. Pharmaceutical-grade chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: The ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Ann. Rheum. Dis. 2017, 76, 1537–1543. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Feng, D.; Xu, L.; Yin, F.; Zang, H.; Liu, C.; Wang, F. Preparation of low molecular weight chondroitin sulfates, screening of a high anti-complement capacity of low molecular weight chondroitin sulfate and its biological activity studies in attenuating osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1685. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Jin, Z. Separation and purification of low-molecular-weight chondroitin sulfates and their anti-oxidant properties. Bangladesh J. Pharmacol. 2016, 11, 61. [Google Scholar] [CrossRef]
- Lan, R.; Li, Y.; Shen, R.; Yu, R.; Jing, L.; Guo, S. Preparation of low-molecular-weight chondroitin sulfates by complex enzyme hydrolysis and their antioxidant activities. Carbohydr. Polym. 2020, 241, 116302. [Google Scholar] [CrossRef]
- Wronski, T.; Morey-Holton, E. Skeletal response to simulated weightlessness: A comparison of suspension techniques. Aviat. Space Environ. Med. 1987, 58, 63–68. [Google Scholar] [PubMed]
- Xue, Y.; Huang, F.; Tang, R.; Fan, Q.; Zhang, B.; Xu, Z.; Sun, X.; Ruan, Z. Chlorogenic acid attenuates cadmium-induced intestinal injury in Sprague-Dawley rats. Food Chem. Toxicol. 2019, 133, 110751. [Google Scholar] [CrossRef] [PubMed]
- Giera, M.; Lingeman, H.; Niessen, W.M. Recent advancements in the LC- and GC-based analysis of malondialdehyde (MDA): A brief overview. Chromatographia 2012, 75, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247. [Google Scholar] [CrossRef]
- Morikawa, D.; Nojiri, H.; Saita, Y.; Kobayashi, K.; Watanabe, K.; Ozawa, Y.; Koike, M.; Asou, Y.; Takaku, T.; Kaneko, K.; et al. Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. J. Bone Miner. Res. 2013, 28, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef]
- Chen, R.M.; Wu, G.J.; Chang, H.C.; Chen, J.T.; Chen, T.F.; Lin, Y.L.; Chen, T.L. 2,6-Diisoprophylphenol protects osteoblasts from oxidative stress-induced apoptosis through suppression of caspase-3 activation. Ann. N. Y. Acad. Sci. 2005, 1042, 448–459. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Igarashi, Y.; Ogawa, T. N-acetyl cysteine protects osteoblastic function from oxidative stress. J. Biomed. Mater. Res. A. 2011, 99, 523–531. [Google Scholar] [CrossRef]
- Sun, Y.; Shuang, F.; Chen, D.M.; Zhou, R.B. Treatment of hydrogen molecule abates oxidative stress and alleviates bone loss induced by modeled microgravity in rats. Osteoporos. Int. 2013, 24, 969–978. [Google Scholar] [CrossRef]
- Malo, M.S. A high level of intestinal alkaline phosphatase is protective against type 2 diabetes mellitus irrespective of obesity. EBioMedicine 2015, 2, 2016–2023. [Google Scholar] [CrossRef]
- Aisha, M.D.; Nor-Ashikin, M.N.; Sharaniza, A.B.; Nawawi, H.M.; Kapitonova, M.Y.; Froemming, G.R. Short-term moderate hypothermia stimulates alkaline phosphatase activity and osteocalcin expression in osteoblasts by upregulating Runx2 and osterix in vitro. Exp. Cell Res. 2014, 326, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.C.; Dutton, J.J.; Piec, I.; Green, D.; Fisher, E.; Washbourne, C.J.; Fraser, W.D. LC–MS/MS application for urine free pyridinoline and free deoxypyridinoline: Urine markers of collagen and bone degradation. Clin. Mass. Spectrom. 2016, 1, 11–18. [Google Scholar] [CrossRef]
- Yan, D.; Bin, C.; Lijun, W.; Zhenyu, W. Polyphenols (S3) isolated from cone scales of pinus koraiensis alleviate decreased bone formation in rat under simulated microgravity. Sci. Rep. 2018, 8, 12719. [Google Scholar]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.; Kensler, T. Nrf2 signaling: An adaptive response pathway for protection against environmental toxic insults. Mutat. Res. 2008, 659, 31–39. [Google Scholar] [CrossRef]
- Wang, R.; Diao, Y.; Kuang, W.; Li, Q.; Tian, Y.; Gao, J.; Dai, L.; Cao, L.; Wang, W.; Wei, L. Salvianolic acid B alleviate the osteoblast activity decreasing under simulated microgravity by Keap1/Nrf2/ARE signaling pathway. J. Funct. Foods. 2018, 46, 288–294. [Google Scholar] [CrossRef]






| Ctrl | HLS | HLS + CS | HLS + LMWCSs | |
|---|---|---|---|---|
| Cancellous bone | ||||
| BMD (g/cm3) | 0.121 ± 0.017 | 0.041 ± 0.003 *** | 0.049 ± 0.004 *** | 0.082 ± 0.006 ***###^^^ |
| BVF (%) | 10.84 ± 1.77 | 1.34 ± 0.34 *** | 1.73 ± 0.12 *** | 6.21 ± 1.01 **##^^ |
| BS/BV (%) | 69.36 ± 2.09 | 111.45 ± 7.70 *** | 103.61 ± 6.00 *** | 86.93 ± 4.72 *##^ |
| Tb. Th (mm) | 0.060 ± 0.003 | 0.042 ± 0.002 *** | 0.045 ± 0.003 ** | 0.051 ± 0.003 *# |
| Tb. N (1/mm) | 1.831 ± 0.373 | 0.321 ± 0.082 *** | 0.388 ± 0.009 *** | 1.143 ± 0.266 *#^ |
| Tb. Sp (mm) | 0.271 ± 0.024 | 0.479 ± 0.081 | 0.481 ± 0.079 | 0.304 ± 0.023 |
| Cortical bone | ||||
| BMD (g/cm3) | 1.407 ± 0.011 | 1.359 ± 0.005 * | 1.343 ± 0.033 | 1.402 ± 0.011 #^ |
| Ct. Th (mm) | 0.247 ± 0.018 | 0.183 ± 0.016 ** | 0.191 ± 0.009 ** | 0.205 ± 0.014 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, R.; Li, Y.; Zhao, X.; Shen, R.; Wang, R.; Mao, R.; Guo, S. Low-Molecular-Weight Chondroitin Sulfates Alleviate Simulated Microgravity-Induced Oxidative Stress and Bone Loss in Mice. Curr. Issues Mol. Biol. 2023, 45, 4214-4227. https://doi.org/10.3390/cimb45050268
Lan R, Li Y, Zhao X, Shen R, Wang R, Mao R, Guo S. Low-Molecular-Weight Chondroitin Sulfates Alleviate Simulated Microgravity-Induced Oxidative Stress and Bone Loss in Mice. Current Issues in Molecular Biology. 2023; 45(5):4214-4227. https://doi.org/10.3390/cimb45050268
Chicago/Turabian StyleLan, Rong, Ye Li, Xinying Zhao, Rong Shen, Ruili Wang, Ruixin Mao, and Shuangsheng Guo. 2023. "Low-Molecular-Weight Chondroitin Sulfates Alleviate Simulated Microgravity-Induced Oxidative Stress and Bone Loss in Mice" Current Issues in Molecular Biology 45, no. 5: 4214-4227. https://doi.org/10.3390/cimb45050268