Gamma-Irradiated Non-Capsule Group B Streptococcus Promotes T-Cell Dependent Immunity and Provides a Cross-Protective Reaction
Abstract
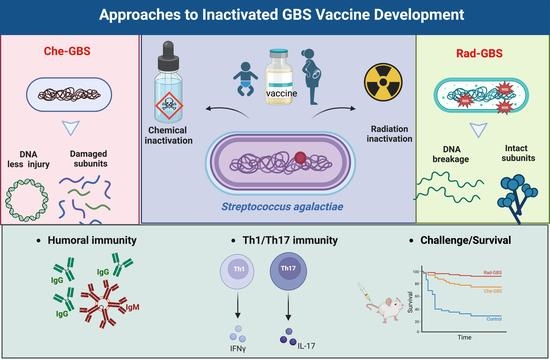
:1. Introduction
2. Results
2.1. Surface Protein Epitope Integrity in Rad-GBS and Che-GBS
2.2. Rad-GBS Induces Maturation of Bone Marrow-Derived DCs
2.3. Rad-GBS Is Highly Immunogenic and Induces an Effective Humoral Immune Response
2.4. Rad-GBS Induces T-Cell Dependent Immune Responses
2.5. Rad-GBS Confers Protection against Hypervirulent Strains in Mice
2.6. Vaccination with Rad-GBS Induces Functional Opsonic Killing Activity and Provides Cross-Protection against Heterologous Serotypes
3. Discussion
4. Materials and Methods
4.1. Bacteria and Culture
4.2. Preparation of Inactivated Bacteria
4.3. Protein Carbonylation Assay
4.4. Analysis of Epitope Integrity by Performing Dot Blots
4.5. Analysis of Mouse Bone Marrow-Derived DC Phenotype
4.6. Mouse Experiment and Ethics
4.7. Determination of Immunoglobulin in Sera
4.8. Opsonophagocytic Killing Assays (OPKAs)
4.9. Flow Cytometry Analysis
4.10. Cytokine Measurements
4.11. Adoptive Transfer of T Cells and Serum into Naïve Mice
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patras, K.A.; Rosler, B.; Thoman, M.L.; Doran, K.S. Characterization of host immunity during persistent vaginal colonization by Group B Streptococcus. Mucosal Immunol. 2015, 8, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Heath, P.T.; Schuchat, A. Perinatal group B streptococcal disease. Best Pract. Research. Clin. Obstet. Gynaecol. 2007, 21, 411–424. [Google Scholar] [CrossRef]
- Russell, N.J.; Seale, A.C.; O’Driscoll, M.; O’Sullivan, C.; Bianchi-Jassir, F.; Gonzalez-Guarin, J.; Lawn, J.E.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. S2), S100–S111. [Google Scholar] [CrossRef] [Green Version]
- Russell, N.J.; Seale, A.C.; O’Sullivan, C.; Le Doare, K.; Heath, P.T.; Lawn, J.E.; Bartlett, L.; Cutland, C.; Gravett, M.; Ip, M.; et al. Risk of Early-Onset Neonatal Group B Streptococcal Disease With Maternal Colonization Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. S2), S152–S159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, N.; Rhodes, J.; Deng, L.; McCarthy, N.; Moline, H.L.; Baggs, J.; Reddy, S.C.; Jernigan, J.A.; Havers, F.P.; Sosin, D.; et al. Changes in the Incidence of Invasive Bacterial Disease During the COVID-19 Pandemic in the United States, 2014–2020. J. Infect. Dis. 2023, jiad028. [Google Scholar] [CrossRef] [PubMed]
- Melin, P. Neonatal group B streptococcal disease: From pathogenesis to preventive strategies. Clin. Microbiol. Infect. 2011, 17, 1294–1303. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.; Adams, N.H.; Bartlett, L.; Seale, A.C.; Lamagni, T.; Bianchi-Jassir, F.; Lawn, J.E.; Baker, C.J.; Cutland, C.; Heath, P.T.; et al. Maternal Disease With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. S2), S112–S124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoff, T.H.; Farley, M.M.; Petit, S.; Craig, A.S.; Schaffner, W.; Gershman, K.; Harrison, L.H.; Lynfield, R.; Mohle-Boetani, J.; Zansky, S.; et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin. Infect. Dis. 2009, 49, 85–92. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO preferred product characteristics for group B streptococcus vaccines. In WHO Preferred Product Characteristics for Group B Streptococcus Vaccines; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hulse, M.L.; Smith, S.; Chi, E.Y.; Pham, A.; Rubens, C.E. Effect of type III group B streptococcal capsular polysaccharide on invasion of respiratory epithelial cells. Infect. Immun. 1993, 61, 4835–4841. [Google Scholar] [CrossRef] [Green Version]
- Dzanibe, S.; Madhi, S.A. Systematic review of the clinical development of group B streptococcus serotype-specific capsular polysaccharide-based vaccines. Expert Rev. Vaccines 2018, 17, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Barro, C.; Salloum, M.; Lim, S.; Delputte, P.; Le Doare, K. Simultaneous carriage of multiple serotypes of Group B Streptococcus: Systematic review and meta-analysis. Vaccine 2023, 41, 15–22. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Bebia, Z.; Maes, C.; Aerssens, A.; De Boever, F.; Grassano, L.; Buffi, G.; Margarit, I.; Karsten, A.; Cho, S.; et al. Safety and Immunogenicity of a Second Dose of an Investigational Maternal Trivalent Group B Streptococcus Vaccine in Nonpregnant Women 4-6 Years After a First Dose: Results From a Phase 2 Trial. Clin. Infect. Dis. 2020, 70, 2570–2579. [Google Scholar] [CrossRef] [Green Version]
- Buurman, E.T.; Timofeyeva, Y.; Gu, J.; Kim, J.H.; Kodali, S.; Liu, Y.; Mininni, T.; Moghazeh, S.; Pavliakova, D.; Singer, C.; et al. A Novel Hexavalent Capsular Polysaccharide Conjugate Vaccine (GBS6) for the Prevention of Neonatal Group B Streptococcal Infections by Maternal Immunization. J. Infect. Dis. 2019, 220, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, P.; Goncalves, B.P.; Le Doare, K.; Lawn, J.E. 20 million pregnant women with group B streptococcus carriage: Consequences, challenges, and opportunities for prevention. Curr. Opin. Pediatr. 2023, 10–1097. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.V.; Ferrieri, P.; Flores, A.E.; Paoletti, L.C. Molecular characterization of nontypeable group B streptococcus. J. Clin. Microbiol. 2006, 44, 2398–2403. [Google Scholar] [CrossRef] [Green Version]
- Kotloff, K.L.; Sztein, M.B.; Wasserman, S.S.; Losonsky, G.A.; DiLorenzo, S.C.; Walker, R.I. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 2001, 69, 3581–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, H.S. Application of radiation technology in vaccines development. Clin. Exp. Vaccine Res. 2015, 4, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Brown, F. Review of accidents caused by incomplete inactivation of viruses. Dev. Biol. Stand. 1993, 81, 103–107. [Google Scholar] [PubMed]
- Kuritzky, L.A.; Pratt, M. Systemic Allergic Contact Dermatitis After Formaldehyde-Containing Influenza Vaccination. J. Cutan. Med. Surg. 2015, 19, 504–506. [Google Scholar] [CrossRef]
- Goldblith, S.A. Radiation sterilization of food. Nature 1966, 210, 433–434. [Google Scholar] [CrossRef]
- Choi, J.I.; Yoon, M.; Joe, M.; Park, H.; Lee, S.G.; Han, S.J.; Lee, P.C. Development of microalga Scenedesmus dimorphus mutant with higher lipid content by radiation breeding. Bioprocess Biosyst. Eng. 2014, 37, 2437–2444. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, J.O.; Lee, S.; Park, J.S.; Gwon, H.J.; Jeong, S.I.; Hardy, J.G.; Lim, Y.M.; Lee, J.Y. Effective gamma-ray sterilization and characterization of conductive polypyrrole biomaterials. Sci. Rep. 2018, 8, 3721. [Google Scholar] [CrossRef] [Green Version]
- Luke, T.C.; Hoffman, S.L. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J. Exp. Biol. 2003, 206 Pt 21, 3803–3808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitman, M.; Tribble, H.R.J.; Green, L. Gamma-irradiated Venezuelan equine encephalitis vaccines. Appl. Microbiol. 1970, 19, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Seong Seo, H.; Ji, H.J.; Yang, E.; Choi, J.A.; Yang, J.S.; Song, M.; Han, S.H.; Lim, S.; Lim, J.H.; et al. Characterization of humoral and cellular immune features of gamma-irradiated influenza vaccine. Hum. Vaccines Immunother. 2020, 17, 485–496. [Google Scholar] [CrossRef]
- Furuya, Y.; Chan, J.; Regner, M.; Lobigs, M.; Koskinen, A.; Kok, T.; Manavis, J.; Li, P.; Mullbacher, A.; Alsharifi, M. Cytotoxic T cells are the predominant players providing cross-protective immunity induced by {gamma}-irradiated influenza A viruses. J. Virol. 2010, 84, 4212–4221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafa, D.; Garg, V.K.; Jain, N.; Sriranganathan, N.; Vemulapalli, R. Immunization of mice with gamma-irradiated Brucella neotomae and its recombinant strains induces protection against virulent B. abortus, B. melitensis, and B. suis challenge. Vaccine 2011, 29, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Secanella-Fandos, S.; Noguera-Ortega, E.; Olivares, F.; Luquin, M.; Julian, E. Killed but metabolically active Mycobacterium bovis bacillus Calmette-Guerin retains the antitumor ability of live bacillus Calmette-Guerin. J. Urol. 2014, 191, 1422–1428. [Google Scholar] [CrossRef]
- Sanakkayala, N.; Sokolovska, A.; Gulani, J.; Hogenesch, H.; Sriranganathan, N.; Boyle, S.M.; Schurig, G.G.; Vemulapalli, R. Induction of antigen-specific Th1-type immune responses by gamma-irradiated recombinant Brucella abortus RB51. Clin. Diagn. Lab. Immunol. 2005, 12, 1429–1436. [Google Scholar] [CrossRef] [Green Version]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S.; et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: Implications for the microbial “pan-genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.S.; Minasov, G.; Seepersaud, R.; Doran, K.S.; Dubrovska, I.; Shuvalova, L.; Anderson, W.F.; Iverson, T.M.; Sullam, P.M. Characterization of fibrinogen binding by glycoproteins Srr1 and Srr2 of Streptococcus agalactiae. J. Biol. Chem. 2013, 288, 35982–35996. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.S.; Mu, R.; Kim, B.J.; Doran, K.S.; Sullam, P.M. Binding of glycoprotein Srr1 of Streptococcus agalactiae to fibrinogen promotes attachment to brain endothelium and the development of meningitis. PLoS Pathog. 2012, 8, e1002947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, H.W. Nontypable group B streptococci isolated from human sources. J. Clin. Microbiol. 1977, 6, 183–184. [Google Scholar] [CrossRef]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Eisen, J.A.; Peterson, S.; Wessels, M.R.; Paulsen, I.T.; Nelson, K.E.; Margarit, I.; Read, T.D.; et al. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. USA 2002, 99, 12391–12396. [Google Scholar] [CrossRef] [Green Version]
- Jang, A.Y.; Choi, M.J.; Zhi, Y.; Ji, H.J.; Noh, J.Y.; Yoon, J.G.; Cheong, H.J.; Kim, W.J.; Seo, H.S.; Song, J.Y. Development and Validation of Enzyme-Linked Immunosorbent Assay for Group B Streptococcal Polysaccharide Vaccine. Vaccines 2021, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.J.; Jang, A.Y.; Song, J.Y.; Ahn, K.B.; Han, S.H.; Bang, S.J.; Jung, H.K.; Hur, J.; Seo, H.S. Development of Live Attenuated Salmonella Typhimurium Vaccine Strain Using Radiation Mutation Enhancement Technology (R-MET). Front. Immunol. 2022, 13, 931052. [Google Scholar] [CrossRef]
- Ingram, L.O. Ethanol tolerance in bacteria. Crit. Rev. Biotechnol. 1990, 9, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Jang, A.Y.; Zhi, Y.; Gao, S.; Lim, S.; Lim, J.H.; Song, J.Y.; Sullam, P.M.; Rhee, J.H.; Seo, H.S. Vaccination With a Latch Peptide Provides Serotype-Independent Protection Against Group B Streptococcus Infection in Mice. J. Infect. Dis. 2017, 217, 93–102. [Google Scholar] [CrossRef]
- Gupta, S. Role of dendritic cells in innate and adaptive immune response in human aging. Exp. Gerontol. 2014, 54, 47–52. [Google Scholar] [CrossRef]
- Gutierrez-Martinez, E.; Planes, R.; Anselmi, G.; Reynolds, M.; Menezes, S.; Adiko, A.C.; Saveanu, L.; Guermonprez, P. Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets. Front. Immunol. 2015, 6, 363. [Google Scholar] [CrossRef] [Green Version]
- Shastri, N.; Schwab, S.; Serwold, T. Producing nature’s gene-chips: The generation of peptides for display by MHC class I molecules. Annu. Rev. Immunol. 2002, 20, 463–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapuente, D.; Storcksdieck Genannt Bonsmann, M.; Maaske, A.; Stab, V.; Heinecke, V.; Watzstedt, K.; Hess, R.; Westendorf, A.M.; Bayer, W.; Ehrhardt, C.; et al. IL-1beta as mucosal vaccine adjuvant: The specific induction of tissue-resident memory T cells improves the heterosubtypic immunity against influenza A viruses. Mucosal Immunol. 2018, 11, 1265–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, F.R.; McLaren, J.E. T Cell Immunity to Bacterial Pathogens: Mechanisms of Immune Control and Bacterial Evasion. Int. J. Mol. Sci. 2020, 21, 6144. [Google Scholar] [CrossRef] [PubMed]
- Le Doare, K.; Heath, P.T. An overview of global GBS epidemiology. Vaccine 2013, 31 (Suppl. S4), D7–D12. [Google Scholar] [CrossRef]
- Slotved, H.C.; Hoffmann, S. Evaluation of procedures for typing of group B Streptococcus: A retrospective study. PeerJ 2017, 5, e3105. [Google Scholar] [CrossRef] [Green Version]
- Teatero, S.; Ferrieri, P.; Martin, I.; Demczuk, W.; McGeer, A.; Fittipaldi, N. Serotype Distribution, Population Structure, and Antimicrobial Resistance of Group B Streptococcus Strains Recovered from Colonized Pregnant Women. J. Clin. Microbiol. 2017, 55, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeage, K. Inactivated quadrivalent split-virus seasonal influenza vaccine (Fluarix(R) quadrivalent): A review of its use in the prevention of disease caused by influenza A and B. Drugs 2013, 73, 1587–1594. [Google Scholar] [CrossRef]
- Uittenbogaard, J.P.; Zomer, B.; Hoogerhout, P.; Metz, B. Reactions of beta-propiolactone with nucleobase analogues, nucleosides, and peptides: Implications for the inactivation of viruses. J. Biol. Chem. 2011, 286, 36198–36214. [Google Scholar] [CrossRef] [Green Version]
- Forster, J.C.; Douglass, M.J.J.; Phillips, W.M.; Bezak, E. Monte Carlo Simulation of the Oxygen Effect in DNA Damage Induction by Ionizing Radiation. Radiat. Res. 2018, 190, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Tuasikal, B.J.; Wibawan, I.W.T.; Pasaribu, F.H.; Estuningsih, S. Bacterial Protein Characterization of Streptococcus agalactiae by SDS-page Method for Subclinical Mastitis Irradiated Vaccine Materials in Dairy Cattle. At. Indones. 2013, 38, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Pasnik, D.J.; Evans, J.J.; Klesius, P.H. A microwave-irradiated Streptococcus agalactiae vaccine provides partial protection against experimental challenge in Nile tilapia, Oreochromis niloticus. World J. Vaccines 2014, 4, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Turner, S.; Raisley, B.; Roach, K.; Bajana, S.; Munroe, M.E.; James, J.A.; Coggeshall, K.M.; Kovats, S. Gram-Positive Bacteria Cell Wall Peptidoglycan Polymers Activate Human Dendritic Cells to Produce IL-23 and IL-1beta and Promote T(H)17 Cell Differentiation. Microorganisms 2023, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Jeong, S.; Jwa, M.Y.; Kim, A.R.; Ha, Y.E.; Kim, S.K.; Jeong, S.; Ahn, K.B.; Seo, H.S.; Yun, C.H.; et al. Immune Responses to Irradiated Pneumococcal Whole Cell Vaccine. Vaccines 2021, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kolls, J.K. T cell-mediated host immune defenses in the lung. Annu. Rev. Immunol. 2013, 31, 605–633. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.J.; Byun, E.B.; Chen, F.; Ahn, K.B.; Jung, H.K.; Han, S.H.; Lim, J.H.; Won, Y.; Moon, J.Y.; Hur, J.; et al. Radiation-Inactivated S. gallinarum Vaccine Provides a High Protective Immune Response by Activating Both Humoral and Cellular Immunity. Front. Immunol. 2021, 12, 717556. [Google Scholar] [CrossRef]
- Clarke, D.; Letendre, C.; Lecours, M.P.; Lemire, P.; Galbas, T.; Thibodeau, J.; Segura, M. Group B Streptococcus Induces a Robust IFN-gamma Response by CD4(+) T Cells in an In Vitro and In Vivo Model. J. Immunol. Res. 2016, 2016, 5290604. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Kim, S.K.; Seo, H.S.; Jeong, S.; Ahn, K.B.; Yun, C.H.; Han, S.H. Th17 activation by dendritic cells stimulated with gamma-irradiated Streptococcus pneumoniae. Mol. Immunol. 2018, 101, 344–352. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Liu, X.; Tang, J.; Peng, B.; Wei, Y. X-ray Irradiated Vaccine Confers protection against Pneumonia caused by Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 18823. [Google Scholar] [CrossRef] [Green Version]
- Lundgren, A.; Bhuiyan, T.R.; Novak, D.; Kaim, J.; Reske, A.; Lu, Y.J.; Qadri, F.; Malley, R. Characterization of Th17 responses to Streptococcus pneumoniae in humans: Comparisons between adults and children in a developed and a developing country. Vaccine 2012, 30, 3897–3907. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, B.; Guo, Y.; Li, W.; Tian, Y.; Sonnenberg, G.F.; Weiser, J.N.; Ni, X.; Shen, H. Cross-protective mucosal immunity mediated by memory Th17 cells against Streptococcus pneumoniae lung infection. Mucosal Immunol. 2017, 10, 250–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olliver, M.; Hiew, J.; Mellroth, P.; Henriques-Normark, B.; Bergman, P. Human monocytes promote Th1 and Th17 responses to Streptococcus pneumoniae. Infect. Immun. 2011, 79, 4210–4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, R.S.W. A Narrative Review of the Molecular Epidemiology and Laboratory Surveillance of Vaccine Preventable Bacterial Meningitis Agents: Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae and Streptococcus agalactiae. Microorganisms 2021, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, D.O.; Mentele, L.M.; Rubens, C.E. Sialylation of group B streptococcal capsular polysaccharide is mediated by cpsK and is required for optimal capsule polymerization and expression. J. Bacteriol. 2005, 187, 4615–4626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.; Rossner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef]
- Choi, M.J.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Lin, S.M.; Zhi, Y.; Lim, J.H.; Lim, S.; Seo, H.S.; Song, J.Y. Development of a multiplexed opsonophagocytic killing assay (MOPA) for group B Streptococcus. Hum. Vaccines Immunother. 2018, 14, 67–73. [Google Scholar] [CrossRef] [Green Version]







| Name | Serotype | Characteristics | Reference or Source |
|---|---|---|---|
| A909 | Ia | Wild-type | [31] |
| COH1 | III | Wild-type | [31] |
| NEM316 ΔSrr1 | III | Wild-type, serine-rich repeat 1 protein knockout | [32] |
| NCTC 1/82 | IV | Wild-type | [33] |
| CNCTC 10/84 | V | Wild-type, hypervirulent | [34] |
| 2603 V/R | V | Wild-type | [35] |
| NSP14-358 | Non-typeable | Clinical isolate from urine of a 74-year-old female with levofloxacin-resistant infection | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, Y.; Chen, F.; Cao, G.; Li, F. Gamma-Irradiated Non-Capsule Group B Streptococcus Promotes T-Cell Dependent Immunity and Provides a Cross-Protective Reaction. Pharmaceuticals 2023, 16, 321. https://doi.org/10.3390/ph16020321
Zhi Y, Chen F, Cao G, Li F. Gamma-Irradiated Non-Capsule Group B Streptococcus Promotes T-Cell Dependent Immunity and Provides a Cross-Protective Reaction. Pharmaceuticals. 2023; 16(2):321. https://doi.org/10.3390/ph16020321
Chicago/Turabian StyleZhi, Yong, Fengjia Chen, Guangxu Cao, and Fang Li. 2023. "Gamma-Irradiated Non-Capsule Group B Streptococcus Promotes T-Cell Dependent Immunity and Provides a Cross-Protective Reaction" Pharmaceuticals 16, no. 2: 321. https://doi.org/10.3390/ph16020321