Anti-Inflammatory Effects of Ang-(1-7) Bone-Targeting Conjugate in an Adjuvant-Induced Arthritis Rat Model
Abstract
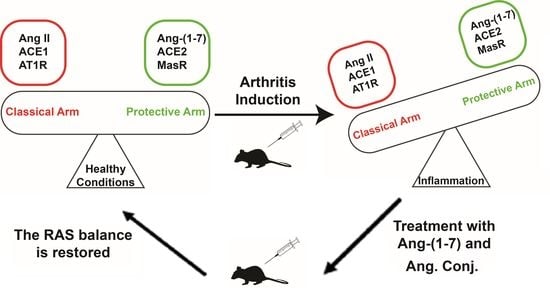
:1. Introduction
2. Results
2.1. Ang. Conj. Treatment Improved Body Weight Gain and Reduced Paw and Joint Swelling
2.2. The Arthritis-Induced High Serum Nitrate and Nitrite Levels Were Reduced after Treatment with Ang-(1-7) and Ang. Conj.
2.3. Treatment with Ang-(1-7) or Ang. Conj. Increased Ang-(1-7) and Reduced Ang II Peptides Levels in Plasma
2.4. Treatment with Ang-(1-7) or Ang. Conj. Reversed the AIA-Induced Changes in ACE1, ACE2, MasR, and AT1R Gene Expression in Different Tissues
2.5. Treatment with Ang-(1-7) or Ang. Conj. Reversed the AIA-Induced Changes in ACE1, ACE2, MasR, and AT1R Protein Expression in Different Tissues
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Arthritis Induction in Rats
4.3. Body Weight, Paw, and Joint Diameter Measurement
4.4. Animal Dosage Regimens, Treatment, and Sampling
4.5. Measurement of the Serum NO Concentration
4.6. Ang Peptides Extraction and Quantification Using Liquid-Chromatography in Tandem with Mass Spectrometry (LC-MS/MS)
4.7. Quantitative Polymerase Chain Reaction (qPCR)
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, v3–v11. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J. Cardiovascular complications of rheumatoid arthritis: Assessment, prevention, and treatment. Rheum. Dis. Clin. 2010, 36, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Heinlen, L.; Humphrey, M. Skeletal complications of rheumatoid arthritis. Osteoporos. Int. 2017, 28, 2801–2812. [Google Scholar] [CrossRef]
- Majithia, V.; Geraci, S.A. Rheumatoid arthritis: Diagnosis and management. Am. J. Med. 2007, 120, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Cobankara, V.; Öztürk, M.A.; Kiraz, S.; Ertenli, I.; Haznedaroglu, I.C.; Pay, S.; Çalgüneri, M. Renin and angiotensin-converting enzyme (ACE) as active components of the local synovial renin-angiotensin system in rheumatoid arthritis. Rheumatol. Int. 2005, 25, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.R.C.; de Oliveira, T.A.; Ramos, N.E.; Abreu, M.A.D.; Simões e Silva, A.C. The role of renin angiotensin system in the pathophysiology of rheumatoid arthritis. Mol. Biol. Rep. 2021, 48, 6619–6629. [Google Scholar] [CrossRef]
- Gonzalez, A.; Icen, M.; Kremers, H.M.; Crowson, C.S.; Davis, J.M.; Therneau, T.M.; Roger, V.L.; Gabriel, S.E. Mortality trends in rheumatoid arthritis: The role of rheumatoid factor. J. Rheumatol. 2008, 35, 1009–1014. [Google Scholar]
- Gabriel, S.E. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am. J. Med. 2008, 121, S9–S14. [Google Scholar] [CrossRef]
- Kaplan, M.J. Cardiovascular disease in rheumatoid arthritis. Curr. Opin. Rheumatol. 2006, 18, 289–297. [Google Scholar] [CrossRef]
- Wei, C.-C.; Tian, B.; Perry, G.; Meng, Q.C.; Chen, Y.-F.; Oparil, S.; Dell’Italia, L.J. Differential ANG II generation in plasma and tissue of mice with decreased expression of the ACE gene. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H2254–H2258. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, K.D.; Coelho, F.M.; Vieira, A.T.; Sachs, D.; Barroso, L.C.; Costa, V.V.; Bretas, T.L.B.; Bader, M.; de Sousa, L.P.; da Silva, T.A. Anti-inflammatory effects of the activation of the angiotensin-(1–7) receptor, MAS, in experimental models of arthritis. J. Immunol. 2010, 185, 5569–5576. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Montezano, A.C. Angiotensin-(1–7) and vascular function: The clinical context. Hypertension 2018, 71, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Barroso, L.C.; Magalhaes, G.S.; Galvão, I.; Reis, A.C.; Souza, D.G.; Sousa, L.P.; Santos, R.A.; Campagnole-Santos, M.J.; Pinho, V.; Teixeira, M.M. Angiotensin-(1-7) promotes resolution of neutrophilic inflammation in a model of antigen-induced arthritis in mice. Front. Immunol. 2017, 8, 1596. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. New Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Pan, W.; Li, Y. Angiotensin-(1–7) attenuates collagen-induced arthritis via inhibiting oxidative stress in rats. Amino Acids 2021, 53, 171–181. [Google Scholar] [CrossRef]
- Connor, J.R.; Manning, P.T.; Settle, S.L.; Moore, W.M.; Jerome, G.M.; Webber, R.K.; Tjoeng, F.S.; Currie, M.G. Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase. Eur. J. Pharmacol. 1995, 273, 15–24. [Google Scholar] [CrossRef]
- Fletcher, D.S.; Widmer, W.R.; Luell, S.; Christen, A.; Orevillo, C.; Shah, S.; Visco, D. Therapeutic administration of a selective inhibitor of nitric oxide synthase does not ameliorate the chronic inflammation and tissue damage associated with adjuvant-induced arthritis in rats. J. Pharmacol. Exp. Ther. 1998, 284, 714–721. [Google Scholar]
- Stefanovic-Racic, M.; Meyers, K.; Meschter, C.; Coffey, J.; Hoffman, R.; Evans, C. Comparison of the nitric oxide synthase inhibitors methylarginine and aminoguanidine as prophylactic and therapeutic agents in rat adjuvant arthritis. J. Rheumatol. 1995, 22, 1922–1928. [Google Scholar]
- McCartney-Francis, N.; Allen, J.B.; Mizel, D.E.; Albina, J.E.; Xie, Q.; Nathan, C.F.; Wahl, S.M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J. Exp. Med. 1993, 178, 749–754. [Google Scholar] [CrossRef]
- Yonekura, Y.; Koshiishi, I.; Yamada, K.-i.; Mori, A.; Uchida, S.; Nakamura, T.; Utsumi, H. Association between the expression of inducible nitric oxide synthase by chondrocytes and its nitric oxide-generating activity in adjuvant arthritis in rats. Nitric Oxide 2003, 8, 164–169. [Google Scholar] [CrossRef]
- Villalobos, L.A.; San Hipólito-Luengo, Á.; Ramos-González, M.; Cercas, E.; Vallejo, S.; Romero, A.; Romacho, T.; Carraro, R.; Sánchez-Ferrer, C.F.; Peiró, C. The angiotensin-(1-7)/mas axis counteracts angiotensin II-dependent and-independent pro-inflammatory signaling in human vascular smooth muscle cells. Front. Pharmacol. 2016, 7, 482. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C.; Pirro, N.T.; Sykes, A.; Ferrario, C.M. Metabolism of angiotensin-(1–7) by angiotensin-converting enzyme. Hypertension 1998, 31, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Mordwinkin, N.M.; Russell, J.R.; Burke, A.S.; Dizerega, G.S.; Louie, S.G.; Rodgers, K.E. Toxicological and toxicokinetic analysis of angiotensin (1–7) in two species. J. Pharm. Sci. 2012, 101, 373–380. [Google Scholar] [CrossRef]
- Aghazadeh-Habashi, A.; Khajehpour, S. Improved pharmacokinetics and bone tissue accumulation of Angiotensin-(1–7) peptide through bisphosphonate conjugation. Amino Acids 2021, 53, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, H.; Fujisaki, J. Bone-specific drug delivery systems. Clin. Pharmacokinet. 2003, 42, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Cawthray, J.; Wasan, E.; Wasan, K. Bone-seeking agents for the treatment of bone disorders. Drug delivery and translational research 2017, 7, 466–481. [Google Scholar] [CrossRef]
- Yang, Y.; Aghazadeh-Habashi, A.; Panahifar, A.; Wu, Y.; Bhandari, K.H.; Doschak, M.R. Bone-targeting parathyroid hormone conjugates outperform unmodified PTH in the anabolic treatment of osteoporosis in rats. Drug Deliv. Transl. Res. 2017, 7, 482–496. [Google Scholar] [CrossRef]
- Bhandari, K.H.; Asghar, W.; Newa, M.; Jamali, F.; Doschak, M.R. Evaluation of bone targeting salmon calcitonin analogues in rats developing osteoporosis and adjuvant arthritis. Curr. Drug Deliv. 2015, 12, 98–107. [Google Scholar] [CrossRef]
- Doschak, M.R.; Kucharski, C.M.; Wright, J.E.; Zernicke, R.F.; Uludag, H. Improved bone delivery of osteoprotegerin by bisphosphonate conjugation in a rat model of osteoarthritis. Mol. Pharm. 2009, 6, 634–640. [Google Scholar] [CrossRef]
- Farrell, A.; Blake, D.; Palmer, R.; Moncada, S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis. 1992, 51, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Leung, B.P.; Field, M.; Wei, X.Q.; Huang, F.-P.; Sturrock, R.D.; Kinninmonth, A.; Weidner, J.; Mumford, R.; Liew, F.Y. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J. Exp. Med. 1996, 184, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Ialenti, A.; Ianaro, A.; Moncada, S.; Di Rosa, M. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol. 1992, 211, 177–182. [Google Scholar] [CrossRef]
- Asghar, W.; Aghazadeh-Habashi, A.; Jamali, F. Cardiovascular effect of inflammation and nonsteroidal anti-inflammatory drugs on renin–angiotensin system in experimental arthritis. Inflammopharmacology 2017, 25, 543–553. [Google Scholar] [CrossRef]
- Maradit-Kremers, H.; Nicola, P.J.; Crowson, C.S.; Ballman, K.V.; Gabriel, S.E. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2005, 52, 722–732. [Google Scholar] [CrossRef]
- Lemarié, C.A.; Schiffrin, E.L. The angiotensin II type 2 receptor in cardiovascular disease. J. Renin-Angiotensin-Aldosterone Syst. 2010, 11, 19–31. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Lorenzo, O.; Suzuki, Y.; Rupérez, M.; Egido, J. Proinflammatory actions of angiotensins. Curr. Opin. Nephrol. Hypertens. 2001, 10, 321–329. [Google Scholar] [CrossRef]
- Kortekaas, K.E.; Meijer, C.A.; Hinnen, J.W.; Dalman, R.L.; Xu, B.; Hamming, J.F.; Lindeman, J.H. ACE inhibitors potently reduce vascular inflammation, results of an open proof-of-concept study in the abdominal aortic aneurysm. PLoS ONE 2014, 9, e111952. [Google Scholar] [CrossRef]
- Benicky, J.; Sánchez-Lemus, E.; Pavel, J.; Saavedra, J.M. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell. Mol. Neurobiol. 2009, 29, 781–792. [Google Scholar] [CrossRef]
- Taguchi, I.; Toyoda, S.; Takano, K.; Arikawa, T.; Kikuchi, M.; Ogawa, M.; Abe, S.; Node, K.; Inoue, T. Irbesartan, an angiotensin receptor blocker, exhibits metabolic, anti-inflammatory and antioxidative effects in patients with high-risk hypertension. Hypertens. Res. 2013, 36, 608–613. [Google Scholar] [CrossRef]
- Price, A.; Lockhart, J.; Ferrell, W.; Gsell, W.; McLean, S.; Sturrock, R. Angiotensin II type 1 receptor as a novel therapeutic target in rheumatoid arthritis: In vivo analyses in rodent models of arthritis and ex vivo analyses in human inflammatory synovitis. Arthritis Rheum. 2007, 56, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Mateo, T.; Nabah, Y.N.A.; Taha, M.A.; Mata, M.; Cerdá-Nicolás, M.; Proudfoot, A.E.; Stahl, R.A.; Issekutz, A.C.; Cortijo, J.; Morcillo, E.J. Angiotensin II-induced mononuclear leukocyte interactions with arteriolar and venular endothelium are mediated by the release of different CC chemokines. J. Immunol. 2006, 176, 5577–5586. [Google Scholar] [CrossRef] [PubMed]
- Nabah, Y.N.A.; Mateo, T.; Estellés, R.; Mata, M.; Zagorski, J.; Sarau, H.; Cortijo, J.; Morcillo, E.J.; Jose, P.J.; Sanz, M.-J. Angiotensin II induces neutrophil accumulation in vivo through generation and release of CXC chemokines. Circulation 2004, 110, 3581–3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlüter, K.-D.; Wenzel, S. Angiotensin II: A hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacol. Ther. 2008, 119, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, N.; Ramprasath, T.; Patel, V.B.; Wang, W.; Putko, B.; Mori, J.; Oudit, G.Y. Targeting angiotensin-converting enzyme 2 as a new therapeutic target for cardiovascular diseases. Can. J. Physiol. Pharmacol. 2014, 92, 558–565. [Google Scholar] [CrossRef]
- Xianwei, W.; Magomed, K.; Ding, Z.; Sona, M.; Jingjun, L.; Shijie, L.; Mehta, J.L. Cross-talk between inflammation and angiotensin II: Studies based on direct transfection of cardiomyocytes with AT1R and AT2R cDNA. Exp. Biol. Med. 2012, 237, 1394–1401. [Google Scholar] [CrossRef]
- Ling, S.; Jamali, F. Effect of early phase adjuvant arthritis on hepatic P450 enzymes and pharmacokinetics of verapamil: An alternative approach to the use of an animal model of inflammation for pharmacokinetic studies. Drug Metab. Dispos. 2005, 33, 579–586. [Google Scholar] [CrossRef]
- Aghazadeh-Habashi, A.; Kohan, M.G.; Asghar, W.; Jamali, F. Glucosamine dose/concentration-effect correlation in the rat with adjuvant arthritis. J. Pharm. Sci. 2014, 103, 760–767. [Google Scholar] [CrossRef]
- Cui, L.; Nithipatikom, K.; Campbell, W.B. Simultaneous analysis of angiotensin peptides by LC–MS and LC–MS/MS: Metabolism by bovine adrenal endothelial cells. Anal. Biochem. 2007, 369, 27–33. [Google Scholar] [CrossRef]
- Dai, S.-Y.; Peng, W.; Zhang, Y.-P.; Li, J.-D.; Shen, Y.; Sun, X.-F. Brain endogenous angiotensin II receptor type 2 (AT2-R) protects against DOCA/salt-induced hypertension in female rats. J. Neuroinflammation 2015, 12, 47. [Google Scholar] [CrossRef]
- Liu, C.X.; Hu, Q.; Wang, Y.; Zhang, W.; Ma, Z.Y.; Feng, J.B.; Wang, R.; Wang, X.P.; Dong, B.; Gao, F. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: A comparison with ACE inhibition. Mol. Med. 2011, 17, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Q.; Liu, S.; Yang, X.; Zhang, Y.; Huang, F. Sini decoction alleviates E. coli induced acute lung injury in mice via equilibrating ACE-AngII-AT1R and ACE2-Ang-(1-7)-Mas axis. Life Sci. 2018, 208, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Exner, E.C.; Geurts, A.M.; Hoffmann, B.R.; Casati, M.; Stodola, T.; Dsouza, N.R.; Zimmermann, M.; Lombard, J.H.; Greene, A.S. Interaction between Mas1 and AT1RA contributes to enhancement of skeletal muscle angiogenesis by angiotensin-(1-7) in Dahl salt-sensitive rats. PLoS ONE 2020, 15, e0232067. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lian, G.; Cai, X.; Lin, Z.; Xie, L. Effect of prehypertensive losartan therapy on AT1R and ATRAP methylation of adipose tissue in the later life of high-fat-fed spontaneously hypertensive rats. Mol. Med. Rep. 2018, 17, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, S.; Dagenais, N.; Dryden, W.; Jamali, F. Effects of angiotensin II blockade on inflammation-induced alterations of pharmacokinetics and pharmacodynamics of calcium channel blockers. Br. J. Pharmacol. 2008, 153, 90–99. [Google Scholar] [CrossRef] [Green Version]






| Paw Diameter Percentage Change Mean (SEM) | Joint Diameter Percentage Change Mean (SEM) | |||
|---|---|---|---|---|
| Animal Group | Left Hind | Right Hind | Left Hind | Right Hind |
| Control (n = 6) | 2.7 (2.0) a | 0.0 (1.3) a | 2.7 (1.2) a | 3.8 (0.8) a |
| Inflamed (n = 6) | 22.7 (10.6) a | 23.7 (10.5) a | 29.0 (9.0) b | 27.7 (7.4) b |
| Ang-(1-7) (n = 5) | 4.8 (1.3) a | 10.0 (10.7) a | 9.6 (2.5) ab | 12.4 (4.6) a |
| Ang. Conj. (n = 5) | 3.1 (1.5) a | 1.8 (0.6) a | 3.6 (1.6) a | 2.5 (1.4) a |
| Gene | Primers | Sequences | Reference |
|---|---|---|---|
| ACE1 | Forward (5′→3′) | TTTGCTACACAAATGGCACTTGT | [50] |
| Reverse (5′→3′) | CGGGACGTGGCCATTATATT | ||
| ACE2 | Forward (5′→3′) | ACCCTTCTTACATCAGCCCTACTG | [51] |
| Reverse (5′→3′) | TGTCCAAAACCTACCCCACATAT | ||
| MasR | Forward (5′→3′) | AGAAATCCCTTCACGGTCTACA | [52] |
| Reverse (5′→3′) | GTCACCGATAATGTCACGATTGT | ||
| AT1R | Forward (5′→3′) | CCTCTACAGCATCATCTTTGTGG | [53] |
| Reverse (5′→3′) | CACACTGGCGTAGAGGTTGA | ||
| GAPDH | Forward (5′→3′) | CCTGCACCACCAACTGCTTA | [54] |
| Reverse (5′→3′) | AGTGATGGCATGGACTGTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khajeh pour, S.; Ranjit, A.; Summerill, E.L.; Aghazadeh-Habashi, A. Anti-Inflammatory Effects of Ang-(1-7) Bone-Targeting Conjugate in an Adjuvant-Induced Arthritis Rat Model. Pharmaceuticals 2022, 15, 1157. https://doi.org/10.3390/ph15091157
Khajeh pour S, Ranjit A, Summerill EL, Aghazadeh-Habashi A. Anti-Inflammatory Effects of Ang-(1-7) Bone-Targeting Conjugate in an Adjuvant-Induced Arthritis Rat Model. Pharmaceuticals. 2022; 15(9):1157. https://doi.org/10.3390/ph15091157
Chicago/Turabian StyleKhajeh pour, Sana, Arina Ranjit, Emma L. Summerill, and Ali Aghazadeh-Habashi. 2022. "Anti-Inflammatory Effects of Ang-(1-7) Bone-Targeting Conjugate in an Adjuvant-Induced Arthritis Rat Model" Pharmaceuticals 15, no. 9: 1157. https://doi.org/10.3390/ph15091157