Investigation of Patient-Centric 3D-Printed Orodispersible Films Containing Amorphous Aripiprazole
Abstract
:1. Introduction
2. Results and Discussion
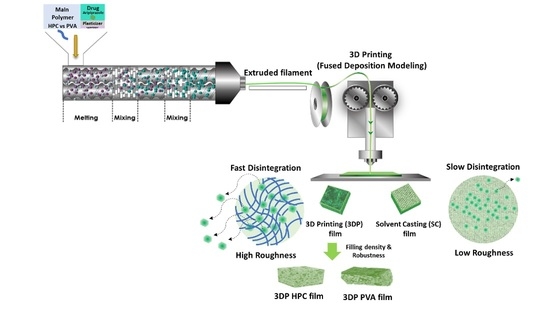
2.1. HME Filament and 3D Printing
2.2. Physicochemical Properties of the ODFs
2.3. Mechanical Properties of the ODFs
2.4. Morphological Examination of the ODFs
2.5. Disintegration Time and Dissolution Profiles of the ODFs
3. Materials and Methods
3.1. Materials
3.2. Preparation of Filaments and Film Using the 3D Printing Method
3.3. Preparation of Film Using the Solvent Casting Method
3.4. Physicochemical Properties of the ODFs
3.5. Morphological Examination of the ODFs
3.6. Mechanical Properties of the ODFs
3.7. HPLC Analysis
3.8. In Vitro Disintegration Studies of the ODFs
3.9. In Vitro Release Studies of the ODFs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krause, J.; Bogdahn, M.; Schneider, F.; Koziolek, M.; Weitschies, W. Design and characterization of a novel 3D printed pressure-controlled drug delivery system. Eur. J. Pharm. Sci. 2019, 140, 105060. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B-Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Yang, Y.; Jia, D.; Li, P.; Li, Q.; Chen, F.; Wang, S.; Pan, W.; Ding, P. Effect of novel internal structures on printability and drug release behavior of 3D printed tablets. J. Drug Deliv. Sci. Technol. 2019, 49, 14–23. [Google Scholar] [CrossRef]
- Pravin, S.; Sudhir, A. Integration of 3D printing with dosage forms: A new perspective for modern healthcare. Biomed. Pharmacother. 2018, 107, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef]
- Thanawuth, K.; Sutthapitaksakul, L.; Konthong, S.; Suttiruengwong, S.; Huanbutta, K.; Dass, C.R.; Sriamornsak, P. Impact of Drug Loading Method on Drug Release from 3D-Printed Tablets Made from Filaments Fabricated by Hot-Melt Extrusion and Impregnation Processes. Pharmaceutics 2021, 13, 1607. [Google Scholar] [CrossRef]
- Chamberlain, R.; Windolf, H.; Geissler, S.; Quodbach, J.; Breitkreutz, J. Precise Dosing of Pramipexole for Low-Dosed Filament Production by Hot Melt Extrusion Applying Various Feeding Methods. Pharmaceutics 2022, 14, 216. [Google Scholar] [CrossRef]
- Hu, X.Y.; Lou, H.; Hageman, M.J. Preparation of lapatinib ditosylate solid dispersions using solvent rotary evaporation and hot melt extrusion for solubility and dissolution enhancement. Int. J. Pharm. 2018, 552, 154–163. [Google Scholar] [CrossRef]
- Ilyes, K.; Kovacs, N.K.; Balogh, A.; Borbas, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuta, I.; Nagy, Z.K. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations-printability-process modulation, with consecutive effects on in vitro release, stability and degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef]
- Tan, D.C.T.; Ong, J.J.; Gokhale, R.; Heng, P.W.S. Hot melt extrusion of ion-exchange resin for taste masking. Int. J. Pharm. 2018, 547, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Palekar, S.; Nukala, P.K.; Patel, K. Aversion liquid-filled drug releasing capsule (3D-RECAL): A novel technology for the development of immediate release abuse deterrent formulations using a fused deposition modelling (FDM) 3D printer. Int. J. Pharm. 2022, 621, 121804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, P.; Chung, S.; Bandari, S.; Repka, M.A. Fabrication of bilayer tablets using hot melt extrusion-based dual-nozzle fused deposition modeling 3D printing. Int. J. Pharm. 2022, 624, 121972. [Google Scholar] [CrossRef] [PubMed]
- Moulton, S.E.; Wallace, G.G. 3-dimensional (3D) fabricated polymer based drug delivery systems. J. Control. Release 2014, 193, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, P.; Vo, A.Q.; Bandari, S.; Yang, F.; Durig, T.; Repka, M.A. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 2019, 52, 292–302. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: In vitro and ex vivo evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Kempin, W.; Franz, C.; Koster, L.C.; Schneider, F.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur. J. Pharm. Biopharm. 2017, 115, 84–93. [Google Scholar] [CrossRef]
- Sjoholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franze, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef]
- Krstic, M.; Radojevic, M.; Stojanovic, D.; Radojevic, V.; Uskokovic, P.; Ibric, S. Formulation and characterization of nanofibers and films with carvedilol prepared by electrospinning and solution casting method. Eur. J. Pharm. Sci. 2017, 101, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged drug release properties for orodispersible films by combining hot-melt extrusion and solvent casting methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The Application of 3D Printing in the Formulation of Multilayered Fast Dissolving Oral Films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Swain, K.; Mallick, S.; Lin, Z. Effect of casting solvent on crystallinity of ondansetron in transdermal films. Int. J. Pharm. 2011, 406, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Afrooz, H.; Mohamed, E.M.; Barakh Ali, S.F.; Dharani, S.; Nutan, M.T.H.; Khan, M.A.; Rahman, Z. Salt Engineering of Aripiprazole with Polycarboxylic Acids to Improve Physicochemical Properties. AAPS PharmSciTech 2021, 22, 31. [Google Scholar] [CrossRef]
- Hamideh, A.; Rahman, Z.; Dharani, S.; Khuroo, T.; Mohamed, E.M.; Nutan, M.T.H.; Reddy, I.K.; Khan, M.A. Preparation and characterization of dicarboxylic acids salt of aripiprazole with enhanced physicochemical properties. Pharm. Dev. Technol. 2021, 26, 455–463. [Google Scholar] [CrossRef]
- Mahajan, S.; Singh, D.; Sharma, R.; Singh, G.; Bedi, N. pH-Independent Dissolution and Enhanced Oral Bioavailability of Aripiprazole-Loaded Solid Self-microemulsifying Drug Delivery System. AAPS PharmSciTech 2021, 22, 24. [Google Scholar] [CrossRef]
- Lai, A.; Sahbaz, Y.; Ford, L.; Nguyen, T.-H.; Haque, S.; Williams, H.D.; Benameur, H.; Scammells, P.J.; Porter, C.J.H. Stabilising disproportionation of lipophilic ionic liquid salts in lipid-based formulations. Int. J. Pharm. 2021, 597, 120292. [Google Scholar] [CrossRef]
- Jamroz, W.; Kurek, M.; Czech, A.; Szafraniec, J.; Gawlak, K.; Jachowicz, R. 3D printing of tablets containing amorphous aripiprazole by filaments co-extrusion. Eur. J. Pharm. Biopharm. 2018, 131, 44–47. [Google Scholar] [CrossRef]
- Jamroz, W.; Kurek, M.; Lyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.H.; Park, K. Simple preparation of coated resin complexes and their incorporation into fast-disintegrating tablets. Arch. Pharm. Res. 2010, 33, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Koner, J.S.; Rajabi-Siahboomi, A.R.; Missaghi, S.; Kirby, D.; Perrie, Y.; Ahmed, J.; Mohammed, A.R. Conceptualisation, Development, Fabrication and In Vivo Validation of a Novel Disintegration Tester for Orally Disintegrating Tablets. Sci. Rep. 2019, 9, 12467. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef]
- Schilling, S.U.; Shah, N.H.; Malick, A.W.; Infeld, M.H.; McGinity, J.W. Citric acid as a solid-state plasticizer for Eudragit RS PO. J. Pharm. Pharmacol. 2007, 59, 1493–1500. [Google Scholar] [CrossRef]
- Jani, R.; Patel, D. Hot melt extrusion: An industrially feasible approach for casting orodispersible film. Asian J. Pharm. Sci. 2015, 10, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Song, C.; Noh, I.; Song, S.; Rhee, Y.S. Hot-Melt 3D Extrusion for the Fabrication of Customizable Modified-Release Solid Dosage Forms. Pharmaceutics 2020, 12, 738. [Google Scholar] [CrossRef]
- Zhang, J.; Vo, A.Q.; Feng, X.; Bandari, S.; Repka, M.A. Pharmaceutical Additive Manufacturing: A Novel Tool for Complex and Personalized Drug Delivery Systems. AAPS PharmSciTech 2018, 19, 3388–3402. [Google Scholar] [CrossRef]
- Hwang, I.; Renuka, V.; Lee, J.H.; Weon, K.Y.; Kang, C.Y.; Lee, B.J.; Park, J.B. Preparation of celecoxib tablet by hot melt extrusion technology and application of process analysis technology to discriminate solubilization effect. Pharm. Dev. Technol. 2020, 25, 525–534. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials-Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Zhong, Y.; He, L.; Zhang, Y.; Jing, G.; Li, S.; Wang, J.; He, H.; Tang, X. Morphological and Crystalline Transitions in Monohydrous and Anhydrous Aripiprazole for a Long-Acting Injectable Suspension. AAPS PharmSciTech 2017, 18, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.M.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The impact of the preparation method on the properties of orodispersible films with aripiprazole: Electrospinning vs. casting and 3D printing methods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panraksa, P.; Qi, S.; Udomsom, S.; Tipduangta, P.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P. Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film. Polymers 2021, 13, 3454. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef]
- Stefani, S.; Hönzke, S.; Cuellar Camacho, J.L.; Neumann, F.; Prasad, A.K.; Hedtrich, S.; Haag, R.; Servin, P. Hyperbranched glycerol-based core-amphiphilic branched shell nanotransporters for dermal drug delivery. Polymer 2016, 96, 156–166. [Google Scholar] [CrossRef]
- Feuerbach, T.; Kock, S.; Thommes, M. Characterisation of fused deposition modeling 3D printers for pharmaceutical and medical applications. Pharm. Dev. Technol. 2018, 23, 1136–1145. [Google Scholar] [CrossRef]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Campos, J.C.; Filho, S.C.; Teixeira, M.C.; Martins-Gomes, C.; Zielinska, A.; Carbone, C.; Silva, A.M. 3D printing in the design of pharmaceutical dosage forms. Pharm. Dev. Technol. 2019, 24, 1044–1053. [Google Scholar] [CrossRef]
- Kalosakas, G.; Martini, D. Drug release from slabs and the effects of surface roughness. Int. J. Pharm. 2015, 496, 291–298. [Google Scholar] [CrossRef]
- Speer, I.; Steiner, D.; Thabet, Y.; Breitkreutz, J.; Kwade, A. Comparative study on disintegration methods for oral film preparations. Eur. J. Pharm. Biopharm. 2018, 132, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yan, J.; Ren, L.; Xue, M.; Yuan, Z.; Wang, T.; Yan, Z.; Yin, L.; Yang, L.; Qin, C. Preparation and evaluation of orally disintegrating film containing donepezil for Alzheimer disease. J. Drug Deliv. Sci. Technol. 2019, 54, 101321. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Wong, S.-C.; Abtahi, M.; Chen, P. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Arakawa, Y.; Kawakami, S.; Yamashita, F.; Hashida, M. Effect of low-molecular-weight beta-cyclodextrin polymer on release of drugs from mucoadhesive buccal film dosage forms. Biol. Pharm. Bull. 2005, 28, 1679–1683. [Google Scholar] [CrossRef] [Green Version]
- Lambros, M.; Tran, T.; Fei, Q.; Nicolaou, M. Citric Acid: A Multifunctional Pharmaceutical Excipient. Pharmaceutics 2022, 14, 972. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, A.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics 2021, 13, 1613. [Google Scholar] [CrossRef]
- Jamroz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications-Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Xu, P.; Zhang, J.; Bandari, S.; Repka, M.A. Development of controlled release oral dosages by density gradient modification via three-dimensional (3D) printing and hot-melt extrusion (HME) technology. J. Drug Deliv. Sci. Technol. 2022, 71, 103355. [Google Scholar] [CrossRef]









| Ingredient | HPC-Filament (wt%/wt%) | PVA-Filament (wt%/wt%) |
|---|---|---|
| Aripiprazole (ARP) | 10 | 10 |
| HPC | 85 | 0 |
| PVA | 0 | 85 |
| Citric acid | 4 | 4 |
| Sucralose | 1 | 1 |
| Processing Parameter | HPC-Filament | PVA-Filament |
| Screw speed (RPM) | 100 | 100 |
| Temperature (℃) | 115 | 200 |
| Feed rate (kg/h) | 0.5 | 0.5 |
| Screw configuration | Standard from ThermoFisher | Standard from ThermoFisher |
| Thickness (mm) | Drug Content (%) | Disintegration Time (s) | Puncture Strength (N/mm²) | |
|---|---|---|---|---|
| HPC-3D Film | 0.11 ± 0.02 | 98.07 ± 4.06 | 45 ± 3.5 | 0.65 ± 0.27 |
| PVA-3D Film | 0.11 ± 0.01 | 98.91 ± 3.10 | 63 ±10.2 | 0.93 ± 0.15 |
| HPC-SC Film | 0.12 ± 0.08 | 99.12 ± 2.06 | 62 ± 5.4 | 0.83 ± 0.30 |
| PVA-SC Film | 0.11 ± 0.07 | 98.41 ± 4.12 | 71 ± 12.3 | 1.12 ± 0.42 |
| Marketed Products [45] | ||||
| EclipseFlash | 0.048 ± 0.01 | 0.34 ± 0.02 | ||
| SmartStrip | 0.15 ± 0.02 | 0.40 ± 0.09 | ||
| References Materials [45] | ||||
| Soft Tissue | 0.10 | |||
| Foil | 0.97 | |||
| Paper | 1.32 | |||
| Surface Roughness Parameter | HPC-3D | PVA-3D | HPC-SC | PVA-SC |
|---|---|---|---|---|
| Roughness Average (nm) | 85.007 | 27.748 | 33.274 | 8.748 |
| Roughness Root Mean Square (nm) | 102.868 | 35.025 | 44.310 | 12.117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Park, C.; Song, I.-O.; Lee, B.-J.; Kang, C.-Y.; Park, J.-B. Investigation of Patient-Centric 3D-Printed Orodispersible Films Containing Amorphous Aripiprazole. Pharmaceuticals 2022, 15, 895. https://doi.org/10.3390/ph15070895
Lee J-H, Park C, Song I-O, Lee B-J, Kang C-Y, Park J-B. Investigation of Patient-Centric 3D-Printed Orodispersible Films Containing Amorphous Aripiprazole. Pharmaceuticals. 2022; 15(7):895. https://doi.org/10.3390/ph15070895
Chicago/Turabian StyleLee, Ju-Hyun, Chulhun Park, In-OK Song, Beom-Jin Lee, Chin-Yang Kang, and Jun-Bom Park. 2022. "Investigation of Patient-Centric 3D-Printed Orodispersible Films Containing Amorphous Aripiprazole" Pharmaceuticals 15, no. 7: 895. https://doi.org/10.3390/ph15070895