Bone Tissue Engineering in the Treatment of Bone Defects
Abstract
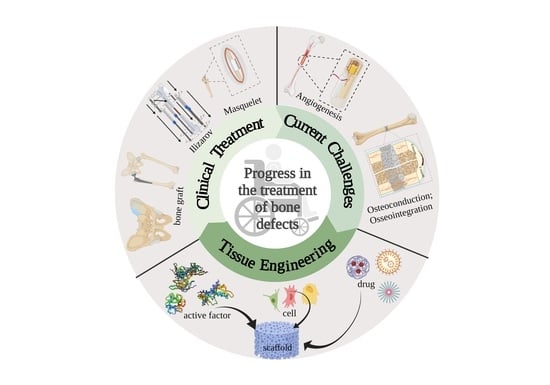
:1. Introduction
2. Advances and Challenges of Bone Defect Treatment
2.1. Clinical Treatment
2.1.1. Bone Grafting
2.1.2. Ilizarov Technique
2.1.3. Masquelet Technique
2.1.4. Bone Graft Substitutes
2.2. Challenges of Bone Defect Treatment
2.2.1. Angiogenesis and Vascularization
2.2.2. Osteoinduction and Osteoconduction
2.2.3. Osseointegration
3. Tissue Engineering Technologies in Bone Regeneration and Repair
3.1. Biomaterials in Bone Tissue Engineering
3.1.1. Inorganic Materials
3.1.2. Natural Biomaterials
3.1.3. Synthetic Polymer
3.2. Cells and Stem Cells in Bone Repair
3.3. Active Factors of Bone Tissue Engineering
3.4. Preparation of Bone Tissue Scaffolds
4. Adjuvant Therapy
4.1. Physiotherapy
4.2. Other Techniques Involved in Bone Tissue Engineering
4.2.1. Exosome
4.2.2. Microneedling
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. Misconceptions (2): Turnover is always higher in cancellous than in cortical bone. Bone 2002, 30, 807–809. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), S131–S139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckstein, F.; Hudelmaier, M.; Putz, R. The effects of exercise on human articular cartilage. J. Anat. 2006, 208, 491–512. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H. Three rules for bone adaptation to mechanical stimuli. Bone 1998, 23, 399–407. [Google Scholar] [CrossRef]
- Martyn-St James, M.; Carroll, S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone 2008, 43, 521–531. [Google Scholar] [CrossRef]
- Watson, S.L.; Weeks, B.K.; Weis, L.J. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: The LIFTMOR randomized controlled trial. J. Bone Miner. Res. 2018, 33, 211–220. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 2010, 41, 27–37. [Google Scholar] [CrossRef]
- Rioja, A.Y.; Daley, E.L.H.; Habif, J.C.; Putnam, A.J.; Stegemann, J.P. Distributed vasculogenesis from modular agarose-hydroxyapatite-fibrinogen microbeads. Acta Biomater. 2017, 55, 144–152. [Google Scholar] [CrossRef]
- Keating, J.F.; Simpson, A.H.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. Br. 2005, 87, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, R.T.; Hong, X.; Schott, N.G.; Tiruchinapally, G.; Levi, B.; Stegemann, J.P. Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects. Biomaterials 2019, 208, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Giannoni, P. Bone Tissue Engineering: Past-Present-Future. Methods Mol. Biol. 2016, 1416, 21–33. [Google Scholar] [PubMed]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salamanca, E.; Hsu, C.C.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Pan, Y.H.; Chang, W.J. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci. Rep. 2018, 8, 984. [Google Scholar] [CrossRef] [Green Version]
- Selhi, H.S.; Mahindra, P.; Yamin, M.; Jain, D.; De Long, W.G., Jr.; Singh, J. Outcome in patients with an infected nonunion of the long bones treated with a reinforced antibiotic bone cement rod. J. Orthop. Trauma 2012, 26, 184–188. [Google Scholar] [CrossRef]
- Malkova, T.A.; Borzunov, D.Y. International recognition of the Ilizarov bone reconstruction techniques: Current practice and research (dedicated to 100(th) birthday of G. A. Ilizarov). World J. Orthop. 2021, 12, 515–533. [Google Scholar] [CrossRef]
- Magadum, M.P.; Basavaraj Yadav, C.M.; Phaneesha, M.S.; Ramesh, L.J. Acute compression and lengthening by the Ilizarov technique for infected nonunion of the tibia with large bone defects. J. Orthop. Surg. 2006, 14, 273–279. [Google Scholar] [CrossRef]
- Rohilla, R.; Sharma, P.K.; Wadhwani, J.; Das, J.; Singh, R.; Beniwal, D. Prospective randomized comparison of bone transport versus Masquelet technique in infected gap nonunion of tibia. Arch. Orthop. Trauma Surg. 2021. [Google Scholar] [CrossRef]
- Walker, M.; Sharareh, B.; Mitchell, S.A. Masquelet Reconstruction for Posttraumatic Segmental Bone Defects in the Forearm. J. Hand Surg. Am. 2019, 44, 342.e1–342.e8. [Google Scholar] [CrossRef]
- Wang, L.J.; Ni, X.H.; Zhang, F.; Peng, Z.; Yu, F.X.; Zhang, L.B.; Li, B.; Jiao, Y.; Li, Y.K.; Yang, B.; et al. Osteoblast Response to Copper-Doped Microporous Coatings on Titanium for Improved Bone Integration. Nanoscale Res. Lett. 2021, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, N.; Da Assuncao, R.; Hancock, N.J.; Lau, A.; Walsh, W.R. Influence of electron beam melting manufactured implants on ingrowth and shear strength in an ovine model. J. Arthroplast. 2012, 27, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Enomoto, Y.; Zhu, W.; Boffelli, M.; Marin, E.; McEntire, B.J. Surface toughness of silicon nitride bioceramics: I, Raman spectroscopy-assisted micromechanics. J. Mech. Behav. Biomed. Mater. 2016, 54, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, P.; Wang, Y.C.; Feng, L.; Xu, R.; Yang, D.B.; Yao, X.H. Studies of Superb Microvascular Imaging and Contrast-Enhanced Ultrasonography in the Evaluation of Vascularization in Early Bone Regeneration. J. Ultrasound Med. 2019, 38, 2963–2971. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cell Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- Thevenot, P.; Nair, A.; Dey, J.; Yang, J.; Tang, L. Method to Analyze Three-Dimensional Cell Distribution and Infiltration in Degradable Scaffolds. Tissue Eng. Part C Methods 2008, 14, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Wang, X.; Jiang, F.; Zhu, Z.; Wen, J.; Jiang, X. Study of Sr-Ca-Si-based scaffolds for bone regeneration in osteoporotic models. Int. J. Oral Sci. 2020, 12, 25. [Google Scholar] [CrossRef]
- Van Houdt, C.I.A.; Koolen, M.K.E.; Lopez-Perez, P.M.; Ulrich, D.J.O.; Jansen, J.A.; Leeuwenburgh, S.C.G.; Weinans, H.H.; van den Beucken, J. Regenerating Critical Size Rat Segmental Bone Defects with a Self-Healing Hybrid Nanocomposite Hydrogel: Effect of Bone Condition and BMP-2 Incorporation. Macromol. Biosci. 2021, 21, e2100088. [Google Scholar] [CrossRef]
- Gurel Pekozer, G.; Abay Akar, N.; Cumbul, A.; Beyzadeoglu, T.; Torun Kose, G. Investigation of Vasculogenesis Inducing Biphasic Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 1526–1538. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontology 2000 2019, 79, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhu, Z.; Peng, F.; Zhang, C.; Xie, J.; Zhou, R.; Zhang, Y.; Li, M. Li-Doped Ti Surface for the Improvement of Osteointegration. ACS Omega 2022, 7, 12030–12038. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, R.P.; Rabelo, G.D.; Sassi, L.M.; Rodrigues, V.P.; Alves, F.A. Implant osseointegration in irradiated bone: An experimental study. J. Periodontal. Res. 2017, 52, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J. Advances in tissue engineering. J. Pediatr. Surg. 2016, 51, 8–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, A.; Saxena, V.; Pandey, L.M. Surface Functionalization of Ti6Al4V via Self-assembled Monolayers for Improved Protein Adsorption and Fibroblast Adhesion. Langmuir 2018, 34, 3494–3506. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Young, G.; Shortkroff, S.; Louie, L.K.; Sledge, C.B.; Yannas, I.V.; Spector, M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials 1997, 18, 769–776. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, K.; Zhou, Y.; Ye, Z.; Tan, W.S. A combinatorial variation in surface chemistry and pore size of three-dimensional porous poly(ε-caprolactone) scaffolds modulates the behaviors of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 193–202. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef]
- Xia, H.; Dong, L.; Hao, M.; Wei, Y.; Duan, J.; Chen, X.; Yu, L.; Li, H.; Sang, Y.; Liu, H. Osteogenic Property Regulation of Stem Cells by a Hydroxyapatite 3D-Hybrid Scaffold with Cancellous Bone Structure. Front. Chem. 2021, 19, 798299. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T. Osseointegrated dental implants. Dent. Clin. N. Am. 1986, 30, 151. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Li, S.; Yi, C.; Zhang, X.; He, Y.; Yu, D. Effect of Pore Size on the Physicochemical Properties and Osteogenesis of Ti6Al4V Porous Scaffolds with Bionic Structure. ACS Omega 2020, 5, 28684–28692. [Google Scholar] [CrossRef]
- Doi, K.; Kobatake, R.; Makihara, Y.; Oki, Y.; Umehara, H.; Kubo, T.; Tsuga, K. The development of novel bioactive porous titanium as a bone reconstruction material. RSC Adv. 2020, 10, 22684–22690. [Google Scholar] [CrossRef]
- Werman, B.S.; Rietschel, R.L. Chronic urticaria from tantalum staples. Arch. Dermatol. 1981, 117, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, A.R.; Patel, D.; Allen, R.K.; Gibson, C.J.; Best, A.M.; Bencharit, S. Retrospective analysis of porous tantalum trabecular metal-enhanced titanium dental implants. J. Prosthet. Dent. 2019, 121, 404–410. [Google Scholar] [CrossRef]
- Wauthle, R.; van der Stok, J.; Amin Yavari, S.; Van Humbeeck, J.; Kruth, J.P.; Zadpoor, A.A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef]
- Sagomonyants, K.B.; Hakim-Zargar, M.; Jhaveri, A.; Aronow, M.S.; Gronowicz, G. Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients. J. Orthop. Res. 2011, 29, 609–616. [Google Scholar] [CrossRef]
- Black, J. Biological performance of tantalum. Clin. Mater. 1994, 16, 167–173. [Google Scholar] [CrossRef]
- Dou, X.; Wei, X.; Liu, G.; Wang, S.; Lv, Y.; Li, J.; Ma, Z.; Zheng, G.; Wang, Y.; Hu, M.; et al. Effect of porous tantalum on promoting the osteogenic differentiation of bone marrow mesenchymal stem cells in vitro through the MAPK/ERK signal pathway. J. Orthop. Translat. 2019, 15, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; He, A.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020, 258, 120280. [Google Scholar] [PubMed]
- Qi, T.; Weng, J.; Yu, F.; Zhang, W.; Li, G.; Qin, H.; Tan, Z.; Zeng, H. Insights into the Role of Magnesium Ions in Affecting Osteogenic Differentiation of Mesenchymal Stem Cells. Biol. Trace Elem. Res. 2021, 199, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Yang, Z.Y.; Tan, L.L.; Li, H.; Zhang, Y.Z. An animal experimental study of porous magnesium scaffold degradation and osteogenesis. Braz. J. Med. Biol. Res. 2014, 47, 715–720. [Google Scholar] [CrossRef]
- Zakhireh, S.; Barar, J.; Adibkia, K.; Beygi-Khosrowshahi, Y.; Fathi, M.; Omidain, H.; Omidi, Y. Bioactive Chitosan-Based Organometallic Scaffolds for Tissue Engineering and Regeneration. Top Curr. Chem. 2022, 380, 13. [Google Scholar] [CrossRef]
- Tanaka, T.; Komaki, H.; Chazono, M.; Kitasato, S.; Kakuta, A.; Akiyama, S.; Marumo, K. Basic research and clinical application of beta-tricalcium phosphate (β-TCP). Morphologie 2017, 101, 164–172. [Google Scholar] [CrossRef]
- Qin, H.; Wei, Y.; Han, J.; Jiang, X.; Yang, X.; Wu, Y.; Gou, Z.; Chen, L. 3D printed bioceramic scaffolds: Adjusting pore dimension is beneficial for mandibular bone defects repair. J. Tissue Eng. Regen. Med. 2022, 16, 409–421. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Yang, K.; Yuan, Y.; Liu, C. RhBMP-2-loaded calcium silicate/calcium phosphate cement scaffold with hierarchically porous structure for enhanced bone tissue regeneration. Biomaterials 2013, 34, 9381–9392. [Google Scholar] [CrossRef]
- Wang, D.; Tabassum, A.; Wu, G.; Deng, L.; Wismeijer, D.; Liu, Y. Bone regeneration in critical-sized bone defect enhanced by introducing osteoinductivity to biphasic calcium phosphate granules. Clin. Oral Implants. Res. 2017, 28, 251–260. [Google Scholar] [CrossRef]
- Montalbano, G.; Borciani, G.; Pontremoli, C.; Ciapetti, G.; Mattioli-Belmonte, M.; Fiorilli, S.; Vitale-Brovarone, C. Development and Biocompatibility of Collagen-Based Composites Enriched with Nanoparticles of Strontium Containing Mesoporous Glass. Materials 2019, 12, 3719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 20, 172–188. [Google Scholar] [CrossRef]
- Banihashemi, M.; Mohkam, M.; Safari, A.; Nezafat, N.; Negahdaripour, M.; Mohammadi, F.; Kianpour, S.; Ghasemi, Y. Optimization of Three-Dimensional Culturing of the HepG2 Cell Line in Fibrin Scaffold. Hepat. Mon. 2015, 15, e22731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soumya, S.; Sreerekha, P.R.; Menon, D.; Nair, S.V.; Chennazhi, K.P. Generation of a biomimetic 3D microporous nano-fibrous scaffold on titanium surfaces for better osteointegration of orthopedic implants. J. Mater. Chem. 2012, 22, 1725–1733. [Google Scholar] [CrossRef]
- Van Houdt, C.I.A.; Ulrich, D.J.O.; Jansen, J.A.; van den Beucken, J. The performance of CPC/PLGA and Bio-Oss (®) for bone regeneration in healthy and osteoporotic rats. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 131–142. [Google Scholar] [CrossRef]
- Rombouts, C.; Jeanneau, C.; Camilleri, J.; Laurent, P.; About, I. Characterization and angiogenic potential of xenogeneic bone grafting materials: Role of periodontal ligament cells. Dent. Mater. J. 2016, 35, 900–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.J.; Bian, Y.F.; Wu, D.; Chen, N.; Wang, L.J. A comparative study of two kinds of artificial bone powder for tooth extraction site preservation. Oral Biomed. 2019, 10, 139–142. [Google Scholar]
- Dong, Y.; Dong, F.J.; Pan, L.F. Effect of Bio-oss bone meal combined with platelet-rich fibrin on mucosal healing and bone defect regeneration after oral implant-guided bone regeneration. Chin. Gen. Pract. 2017, 20, 152–154. [Google Scholar]
- Xu, M.Y.; Liu, Y.H.; Wang, P.S.; Hu, Y.C. Research progress of bioactive glass-based bone repair materials. Chin. J. Orthop. 2019, 12, 440–448. [Google Scholar]
- Jin, S.Y.; Kim, S.G.; Oh, J.S.; You, J.S.; Lim, S.C.; Jeong, M.A.; Kim, J.S. Histomorphometric Analysis of Contaminated Autogenous Tooth Graft Materials after Various Sterilization. Implant Dent. 2016, 25, 83–89. [Google Scholar] [CrossRef]
- Kim, E.S. Autogenous fresh demineralized tooth graft prepared at chairside for dental implant. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sas, A.; Helgason, B.; Ferguson, S.J.; van Lenthe, G.H. Mechanical and morphological characterization of PMMA/bone composites in human femoral heads. J. Mech. Behav. Biomed. Mater. 2021, 115, 104247. [Google Scholar] [CrossRef] [PubMed]
- Hoess, A.; López, A.; Engqvist, H.; Ott, M.K.; Persson, C. Comparison of a quasi-dynamic and a static extraction method for the cytotoxic evaluation of acrylic bone cements. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Properties of nanofiller-loaded poly (methyl methacrylate) bone cement composites for orthopedic applications: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1260–1284. [Google Scholar] [CrossRef]
- Xu, D.; Song, W.; Zhang, J.; Liu, Y.; Lu, Y.; Zhang, X.; Liu, Q.; Yuan, T.; Liu, R. Osteogenic effect of polymethyl methacrylate bone cement with surface modification of lactoferrin. J. Biosci. Bioeng. 2021, 132, 132–139. [Google Scholar] [CrossRef]
- Lin, Y.; Umebayashi, M.; Abdallah, M.N.; Dong, G.; Roskies, M.G.; Zhao, Y.F.; Murshed, M.; Zhang, Z.; Tran, S.D. Combination of polyetherketoneketone scaffold and human mesenchymal stem cells from temporomandibular joint synovial fluid enhances bone regeneration. Sci. Rep. 2019, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Hakkarainen, M.; Albertsson, A.C. Degradation Products of Aliphatic and Aliphatic–Aromatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Ciobanu, P.; Panuta, A.; Radu, I.; Forna, N.; Arcana, S.; Tudor, R.; Covaciu, A.; Niculescu, V.; Poroch, V.; Puha, B. Treatment of Bone Defects Resulted after Excision of Enchondroma of the Hand in 15 Patients, Comparing the Techniques of Autologous Bone Graft, Injectable Bone Substitute and Spontaneous Healing. Appl. Sci. 2022, 12, 1300. [Google Scholar] [CrossRef]
- Jain, G.; Blaauw, D.; Chang, S. A Comparative Study of Two Bone Graft Substitutes—InterOss® Collagen and OCS-B Collagen®. J. Funct. Biomater. 2022, 13, 28. [Google Scholar] [CrossRef]
- Kim, J.-S.; Jang, T.-S.; Kim, S.-Y.; Lee, W.-P. Octacalcium Phosphate Bone Substitute (Bontree®): From Basic Research to Clinical Case Study. Appl. Sci. 2021, 11, 7921. [Google Scholar] [CrossRef]
- Hofmann, A.; Gorbulev, S.; Guehring, T.; Schulz, A.P.; Schupfner, R.; Raschke, M.; Huber-Wagner, S.; Rommens, P.M.; on behalf of the CERTiFy Study Group. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. J. Bone Jt. Surgery. 2020, 102, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, K.F.; Heilig, P.; McDonogh, M.; Boelch, S.; Gbureck, U.; Meffert, R.H.; Hoelscher-Doht, S.; Jordan, M.C. Cement-augmented screw fixation for calcaneal fracture treatment: A biomechanical study comparing two injectable bone substitutes. J. Orthop. Surg. Res. 2020, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Westhauser, F.; Karadjian, M.; Essers, C.; Senger, A.S.; Hagmann, S.; Schmidmaier, G.; Moghaddam, A. Osteogenic differentiation of mesenchymal stem cells is enhanced in a 45S5-supplemented β-TCP composite scaffold: An in-vitro comparison of Vitoss and Vitoss BA. PLoS ONE 2019, 14, e0212799. [Google Scholar] [CrossRef] [PubMed]
- Kent, N.W.; Blunn, G.; Karpukhina, N.; Davis, G.; de Godoy, R.F.; Wilson, R.M.; Coathup, M.; Onwordi, L.; Quak, W.Y.; Hill, R. In vitro and in vivo study of commercial calcium phosphate cement HydroSetTM. J. Biomed. Mater. Res. B 2018, 106, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.T.; Kuo, C.L.; Yeh, T.T.; Shen, H.C.; Pan, R.Y.; Wu, C.C. Modified Essex-Lopresti procedure with percutaneous calcaneoplasty for comminuted intra-articular calcaneal fractures: A retrospective case analysis. BMC Musculoskelet. Dis. 2018, 19, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frasca, S.; Norol, F.; Le Visage, C.; Collombet, J.M.; Letourneur, D.; Holy, X.; Sari Ali, E. Calcium-phosphate ceramics and polysaccharide-based hydrogel scaffolds combined with mesenchymal stem cell differently support bone repair in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 28, 35. [Google Scholar] [CrossRef] [Green Version]
- Duan, R.; Barbieri, D.; Luo, X.; Weng, J.; Bao, C.; de Bruijn, J.D.; Yuan, H. Variation of the bone forming ability with the physicochemical properties of calcium phosphate bone substitutes. Biomater. Sci. 2017, 6, 136–145. [Google Scholar] [CrossRef]
- Mellier, C.; Lefèvre, F.X.; Fayon, F.; Montouillout, V.; Despas, C.; Le Ferrec, M.; Boukhechba, F.; Walcarius, A.; Janvier, P.; Dutilleul, M.; et al. A straightforward approach to enhance the textural, mechanical and biological properties of injectable calcium phosphate apatitic cements (CPCs): CPC/blood composites, a comprehensive study. Acta Biomater. 2017, 62, 328–339. [Google Scholar] [CrossRef]
- Nusselt, T.; Hofmann, A.; Wachtlin, D.; Gorbulev, S.; Rommens, P.M. CERAMENT treatment of fracture defects (CERTiFy): Protocol for a prospective, multicenter, randomized study investigating the use of CERAMENT™ BONE VOID FILLER in tibial plateau fractures. Trials 2014, 15, 75. [Google Scholar] [CrossRef] [Green Version]
- Kassim, B.; Ivanovski, S.; Mattheos, N. Current perspectives on the role of ridge (socket) preservation procedures in dental implant treatment in the aesthetic zone. Aust. Dent. J. 2014, 59, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Gohil, S.V.; Adams, D.J.; Maye, P.; Rowe, D.W.; Nair, L.S. Evaluation of rhBMP-2 and bone marrow derived stromal cell mediated bone regeneration using transgenic fluorescent protein reporter mice. J. Biomed. Mater. Res. A 2014, 102, 4568–4580. [Google Scholar] [CrossRef] [Green Version]
- Dallari, D.; Savarino, L.; Albisinni, U.; Fornasari, P.; Ferruzzi, A.; Baldini, N.; Giannini, S. A prospective, randomised, controlled trial using a Mg-hydroxyapatite—Demineralized bone matrix nanocomposite in tibial osteotomy. Biomaterials 2012, 33, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, S.; Jin, D.; Sheng, J.; Chen, S.; Cheng, X.; Zhang, C. Free vascularised fibular grafting with OsteoSet®2 demineralised bone matrix versus autograft for large osteonecrotic lesions of the femoral head. Int. Orthop. 2011, 35, 475–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hieu, P.D.; Chung, J.H.; Yim, S.B.; Hong, K.S. A radiographical study on the changes in height of grafting materials after sinus lift: A comparison between two types of xenogenic materials. J. Periodontal. Implant Sci. 2010, 40, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Fujishiro, T.; Belkoff, S.M.; Kobayashi, N.; Turner, A.S.; Seim, H.B., 3rd; Zitelli, J.; Hawkins, M.; Bauer, T.W. Long-term evaluation of a calcium phosphate bone cement with carboxymethyl cellulose in a vertebral defect model. J. Biomed. Mater. Res. A 2009, 88, 880–888. [Google Scholar] [CrossRef]
- Van Lieshout, E.M.; Van Kralingen, G.H.; El-Massoudi, Y.; Weinans, H.; Patka, P. Microstructure and biomechanical characteristics of bone substitutes for trauma and orthopaedic surgery. BMC Musculoskelet. Disord. 2011, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Huber, F.X.; McArthur, N.; Heimann, L.; Dingeldein, E.; Cavey, H.; Palazzi, X.; Clermont, G.; Boutrand, J.P. Evaluation of a novel nanocrystalline hydroxyapatite paste Ostim in comparison to Alpha-BSM—More bone ingrowth inside the implanted material with Ostim compared to Alpha BSM. BMC Musculoskelet. Disord. 2009, 10, 164. [Google Scholar] [CrossRef] [Green Version]
- Rauschmann, M.A.; Wichelhaus, T.A.; Stirnal, V.; Dingeldein, E.; Zichner, L.; Schnettler, R.; Alt, V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 2005, 26, 2677–2684. [Google Scholar] [CrossRef]
- Sanus, G.Z.; Tanriverdi, T.; Ulu, M.O.; Kafadar, A.M.; Tanriover, N.; Ozlen, F. Use of Cortoss as an alternative material in calvarial defects: The first clinical results in cranioplasty. J. Craniofacial Surg. 2008, 19, 88–95. [Google Scholar] [CrossRef]
- Boszczyk, B. Prospective study of standalone balloon kyphoplasty with calcium phosphate cement augmentation in traumatic fractures (G. Maestretti et al.). Eur. Spine J. 2007, 16, 611. [Google Scholar] [CrossRef] [Green Version]
- Welch, R.D.; Zhang, H.; Bronson, D.G. Experimental tibial plateau fractures augmented with calcium phosphate cement or autologous bone graft. J. Bone Jt. Surg. Am. 2003, 85, 222–231. [Google Scholar] [CrossRef]
- Zimmermann, R.; Gabl, M.; Lutz, M.; Angermann, P.; Gschwentner, M.; Pechlaner, S. Injectable calcium phosphate bone cement Norian SRS for the treatment of intra-articular compression fractures of the distal radius in osteoporotic women. Arch. Orthop. Trauma Surg. 2003, 123, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsafadi, M.; Manikandan, M.; Atteya, M.; Hashmi, J.A.; Iqbal, Z.; Aldahmash, A.; Alfayez, M.; Kassem, M.; Mahmood, A. Characterization of Cellular and Molecular Heterogeneity of Bone Marrow Stromal Cells. Stem Cells Int. 2016, 2016, 9378081. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Zhao, Y.; Wang, C.; Wang, Z.; Wang, J.; Liu, H.; Feng, Y.; Lin, Q.; Li, Z.; Liu, H. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics 2020, 10, 4779–4794. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Zhao, Z.; Ma, L.; Li, W.; Li, S.; Zhang, J. Cyclic Adenosine Monophosphate-Enhanced Calvarial Regeneration by Bone Marrow-Derived Mesenchymal Stem Cells on a Hydroxyapatite/Gelatin Scaffold. ACS Omega 2021, 6, 13684–13694. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Hutton, D.L.; Grayson, W.L. Stem cell-based approaches to engineering vascularized bone. Curr. Opin. Chem. Eng. 2014, 3, 75–82. [Google Scholar] [CrossRef]
- Li, Y.; Charif, N.; Mainard, D.; Stoltz, J.F.; Isla, N.D. The importance of mesenchymal stem cell donor’s age for cartilage engineering. Osteoarthr. Cartil. 2014, 22, S61. [Google Scholar] [CrossRef] [Green Version]
- Sheykhhasan, M.; Wong, J.K.L.; Seifalian, A.M. Human Adipose-Derived Stem Cells with Great Therapeutic Potential. Curr. Stem Cell Res. Ther. 2019, 14, 532–548. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.H.; Lee, K.M.; Lee, C.K.; Shin, D.M. BMP-2 Induced Signaling Pathways and Phenotypes: Comparisons Between Senescent and Non-senescent Bone Marrow Mesenchymal Stem Cells. Calcif. Tissue Int. 2022, 110, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Whitehead, J.; Yasui, O.W.; Leach, J.K. Bioreactor culture duration of engineered constructs influences bone formation by mesenchymal stem cells. Biomaterials 2017, 146, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Fukunishi, T.; Best, C.A.; Ong, C.S.; Groehl, T.; Reinhardt, J.; Yi, T.; Miyachi, H.; Zhang, H.; Shinoka, T.; Breuer, C.K.; et al. Role of Bone Marrow Mononuclear Cell Seeding for Nanofiber Vascular Grafts. Tissue Eng. Part A 2018, 24, 135–144. [Google Scholar] [CrossRef]
- Mizuno, H. Adipose-derived stem cells for tissue repair and regeneration: Ten years of research and a literature review. J. Nippon Med. Sch. 2009, 76, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, H.; Itoi, Y.; Kawahara, S.; Ogawa, R.; Akaishi, S.; Hyakusoku, H. In vivo adipose tissue regeneration by adipose-derived stromal cells isolated from GFP transgenic mice. Cells Tissues Organs 2008, 187, 177–185. [Google Scholar]
- Tang, M.; Chen, W.; Weir, M.D.; Thein-Han, W.; Xu, H.H. Human embryonic stem cell encapsulation in alginate microbeads in macroporous calcium phosphate cement for bone tissue engineering. Acta Biomater. 2012, 8, 3436–3445. [Google Scholar] [CrossRef] [Green Version]
- Sui, B.; Chen, C.; Kou, X.; Li, B.; Xuan, K.; Shi, S.; Jin, Y. Pulp Stem Cell-Mediated Functional Pulp Regeneration. J. Dent. Res. 2019, 98, 27–35. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Zhang, W.; Zhou, M.; Zhou, Z.; Shen, M.; Chen, N.; Jiang, X. Human amniotic mesenchymal stromal cells promote bone regeneration via activating endogenous regeneration. Theranostics 2020, 10, 6216–6230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Li, D.X.; Zhao, Z.J.; Zhang, C.Y. Research progress of bone tissue engineering in skull repair. Med. Recapitul. 2021, 26, 4817–4823. [Google Scholar]
- Novais, A.; Chatzopoulou, E.; Chaussain, C.; Gorin, C. The Potential of FGF-2 in Craniofacial Bone Tissue Engineering: A Review. Cells 2021, 10, 932. [Google Scholar] [CrossRef]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Marie, P.J. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002, 16, 1446–1465. [Google Scholar] [PubMed] [Green Version]
- Nakamura, Y.; Tensho, K.; Nakaya, H.; Nawata, M.; Okabe, T.; Wakitani, S. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone 2005, 36, 399–407. [Google Scholar] [CrossRef]
- Gronowicz, G.; Jacobs, E.; Peng, T.; Zhu, L.; Hurley, M.; Kuhn, L.T. Calvarial Bone Regeneration Is Enhanced by Sequential Delivery of FGF-2 and BMP-2 from Layer-by-Layer Coatings with a Biomimetic Calcium Phosphate Barrier Layer. Tissue Eng. Part A 2017, 23, 1490–1501. [Google Scholar] [CrossRef]
- García, J.R.; Clark, A.Y.; García, A.J. Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. J. Biomed. Mater. Res. A 2016, 104, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.P.; Raftery, R.M.; Murphy, R.; Chen, G.; Heise, A.; O’Brien, F.J.; Cryan, S.A. Gene activated scaffolds incorporating star-shaped polypeptide-pDNA nanomedicines accelerate bone tissue regeneration in vivo. Biomater. Sci. 2021, 9, 4984–4999. [Google Scholar] [CrossRef]
- Naudot, M.; Garcia Garcia, A.; Jankovsky, N.; Barre, A.; Zabijak, L.; Azdad, S.Z.; Collet, L.; Bedoui, F.; Hébraud, A.; Schlatter, G.; et al. The combination of a poly-caprolactone/nano-hydroxyapatite honeycomb scaffold and mesenchymal stem cells promotes bone regeneration in rat calvarial defects. J. Tissue Eng. Regen. Med. 2020, 14, 1570–1580. [Google Scholar] [CrossRef]
- Ding, R.; Wu, Z.Z.; Qiu, G.X.; Wu, G.; Wang, H.; Su, X.L.; Yin, B.; Ma, S.; Qi, B. Bone tissue engineering observation of porous titanium alloy scaffolds by selective laser sintering. Natl. Med. J. China 2014, 94, 1499–1502. [Google Scholar]
- Ruan, S.Q.; Deng, J.; Yan, L.; Huang, W.L. Composite scaffolds loaded with bone mesenchymal stem cells promote the repair of radial bone defects in rabbit model. Biomed. Pharmacother. 2018, 97, 600–606. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, D.; Wang, M.; Zhang, D.; Xu, Y. Three-Dimensional Printed Titanium Scaffolds Enhance Osteogenic Differentiation and New Bone Formation by Cultured Adipose Tissue-Derived Stem Cells Through the IGF-1R/AKT/Mammalian Target of Rapamycin Complex 1 (mTORC1) Pathway. Med. Sci. Monit. 2019, 25, 8043–8054. [Google Scholar] [CrossRef]
- Kakuta, A.; Tanaka, T.; Chazono, M.; Komaki, H.; Kitasato, S.; Inagaki, N.; Akiyama, S.; Marumo, K. Effects of micro-porosity and local BMP-2 administration on bioresorption of β-TCP and new bone formation. Biomater. Res. 2019, 23, 12. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Yang, S.S.; Kim, C.S. Incorporation of BMP-2 nanoparticles on the surface of a 3D-printed hydroxyapatite scaffold using an ε-polycaprolactone polymer emulsion coating method for bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 170, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xu, Z.; Ke, Q.; Yin, W.; Chen, Y.; Zhang, C.; Guo, Y. Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Ding, T.; Yang, W.Q.; Guo, X.J. Repair of bone defect by nano-hydroxyapatite/chitosan scaffold loaded with xylosaponin D. J. Clin. Rehabil. Tissue Eng. Res. 2022, 26, 4293–4299. [Google Scholar]
- Zhang, H.; Zhou, Y.; Yu, N.; Ma, H.; Wang, K.; Liu, J.; Zhang, W.; Cai, Z.; He, Y. Construction of vascularized tissue-engineered bone with polylysine-modified coral hydroxyapatite and a double cell-sheet complex to repair a large radius bone defect in rabbits. Acta Biomater. 2019, 91, 82–98. [Google Scholar] [CrossRef]
- Wang, W.; Miao, Y.; Zhou, X.; Nie, W.; Chen, L.; Liu, D.; Du, H.; He, C. Local Delivery of BMP-2 from Poly (lactic-co-glycolic acid) Microspheres Incorporated into Porous Nanofibrous Scaffold for Bone Tissue Regeneration. J. Biomed. Nanotechnol. 2017, 13, 1446–1456. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Du, C. Macroporous poly (l-lactic acid)/chitosan nanofibrous scaffolds through cloud point thermally induced phase separation for enhanced bone regeneration. Eur. Polym. J. 2018, 109, 303–316. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, J.; Ding, S.; Li, H.; Zhou, C.; Li, L. In vitro evaluation of random and aligned polycaprolactone/gelatin fibers via electrospinning for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Ma, J.; Ni, S.; Liu, C.; Wang, Y.; Wu, S.; Liu, J.; Weng, Y.; Zhou, D.; Jimenez-Franco, A.; et al. Attapulgite-doped electrospun PCL scaffolds for enhanced bone regeneration in rat cranium defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 133, 112656. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ramiah, P.; Toit, L.; Choonara, Y.E.; Kondia, H.P.; Pillay, V. Hydrogel-Based Bioinks for 3D Bioprinting in Tissue Regeneration. Front. Mater. 2020, 7, 76. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 8, e1801048. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zou, B.; Lai, Q.; Wang, Y.; Xue, R.; Xing, H.; Fu, X.; Huang, C.; Yao, P. A study on biosafety of HAP ceramic prepared by SLA-3D printing technology directly. J. Mech. Behav. Biomed. Mater. 2019, 98, 327–335. [Google Scholar] [CrossRef]
- Yz, A.; Hs, A.; Tao, L.A.; Jiang, P.; Awb, C.; Zz, A.; Lw, A.; Sl, A.; Xy, A. Effect of Ca/P ratios on porous calcium phosphate salt bioceramic scaffolds for bone engineering by 3D gel-printing method—ScienceDirect. Ceram. Int. 2019, 45, 20493–20500. [Google Scholar]
- Zeng, Y.; Zhou, M.; Mou, S.; Yang, J.; Yuan, Q.; Guo, L.; Zhong, A.; Wang, J.; Sun, J.; Wang, Z. Sustained delivery of alendronate by engineered collagen scaffold for the repair of osteoporotic bone defects and resistance to bone loss. J. Biomed. Mater. Res. A 2020, 108, 2460–2472. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; Tan, K.H.; Wiria, F.E.; Cheah, C.M. Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J. Mater. Sci. Mater. Med. 2004, 15, 1113–1121. [Google Scholar] [CrossRef]
- Liu, C.G.; Zeng, Y.T.; Kankala, R.K.; Zhang, S.S.; Chen, A.Z.; Wang, S.B. Characterization and Preliminary Biological Evaluation of 3D-Printed Porous Scaffolds for Engineering Bone Tissues. Materials 2018, 11, 1832. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xuan, P.; Ping, S.; Sun, H.; Li, H.; Fan, Y.; Jiang, Q.; Zhou, C.; Zhang, X. Porous bioceramics produced by inkjet 3D printing: Effect of printing ink formulation on the ceramic macro and micro porous architectures control. Compos. B Eng. 2018, 155, 112–121. [Google Scholar] [CrossRef]
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D Printing of Bioceramic Scaffolds-Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, M.; Pan, N.; Liu, Q.; Ren, X.; Liu, Y.; Huang, T.S. Three-dimensionally printed polylactic acid/cellulose acetate scaffolds with antimicrobial effect. RSC Adv. 2020, 10, 2952–2958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cheng, G.; Xing, X.; Liu, J.; Cheng, Y.; Ye, T.; Wang, Q.; Xiao, X.; Li, Z.; Deng, H. Near-Infrared Light-Triggered Porous AuPd Alloy Nanoparticles To Produce Mild Localized Heat To Accelerate Bone Regeneration. J. Phys. Chem. Lett. 2019, 10, 4185–4191. [Google Scholar] [CrossRef]
- 1Isaacson, B.M.; Bloebaum, R.D. Bone bioelectricity: What have we learned in the past 160 years? J. Biomed. Mater. Res. A 2010, 95, 1270–1279. [Google Scholar] [CrossRef]
- Kuzyk, P.R.; Schemitsch, E.H. The science of electrical stimulation therapy for fracture healing. Indian J. Orthop. 2009, 43, 127–131. [Google Scholar]
- Darendeliler, M.A.; Darendeliler, A.; Sinclair, P.M. Effects of static magnetic and pulsed electromagnetic fields on bone healing. Int. J. Adult Orthodon Orthognath. Surg. 1997, 12, 43–53. [Google Scholar]
- Maharjan, B.; Kaliannagounder, V.K.; Jang, S.R.; Awasthi, G.P.; Bhattarai, D.P.; Choukrani, G.; Park, C.H.; Kim, C.S. In-situ polymerized polypyrrole nanoparticles immobilized poly(ε-caprolactone) electrospun conductive scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111056. [Google Scholar] [CrossRef]
- Yun, H.M.; Ahn, S.J.; Park, K.R.; Kim, M.J.; Kim, J.J.; Jin, G.Z.; Kim, H.W.; Kim, E.C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, D.; Wu, X.; Dang, L.; Lu, A.; Zhang, G. Bone-derived exosomes. Curr. Opin. Pharmacol. 2017, 34, 64–69. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of Small RNA-Modulated Exosome Mimetics for Bone Regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef]
- Xu, J.F.; Yang, G.H.; Pan, X.H.; Zhang, S.J.; Zhao, C.; Qiu, B.S.; Gu, H.F.; Hong, J.F.; Cao, L.; Chen, Y.; et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS ONE 2014, 9, e114627. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Wang, S.; Tang, H.; Li, D.; Zhou, F.; Xin, L.; He, Q.; Hu, S.; Zhang, T.; Chen, T.; et al. Dynamically Bioresponsive DNA Hydrogel Incorporated with Dual-Functional Stem Cells from Apical Papilla-Derived Exosomes Promotes Diabetic Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 16082–16099. [Google Scholar] [CrossRef]
- Li, F.; Wu, J.; Li, D.; Hao, L.; Li, Y.; Yi, D.; Yeung, K.W.K.; Chen, D.; Lu, W.W.; Pan, H.; et al. Engineering stem cells to produce exosomes with enhanced bone regeneration effects: An alternative strategy for gene therapy. J. Nanobiotechnol. 2022, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, H.; Fu, H.; Hu, Y.; Fang, W.; Liu, J. Exosome-delivered BMP-2 and polyaspartic acid promotes tendon bone healing in rotator cuff tear via Smad/RUNX2 signaling pathway. Bioengineered 2022, 13, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- Bin-Bin, Z.; Da-Wa, Z.X.; Chao, L.; Lan-Tao, Z.; Tao, W.; Chuan, L.; Chao-Zheng, L.; De-Chun, L.; Chang, F.; Shu-Qing, W.; et al. M2 macrophagy-derived exosomal miRNA-26a-5p induces osteogenic differentiation of bone mesenchymal stem cells. J. Orthop. Surg. Res. 2022, 17, 137. [Google Scholar] [CrossRef]
- Lin, Z.; Xiong, Y.; Meng, W.; Hu, Y.; Chen, L.; Chen, L.; Xue, H.; Panayi, A.C.; Zhou, W.; Sun, Y.; et al. Exosomal PD-L1 induces osteogenic differentiation and promotes fracture healing by acting as an immunosuppressant. Bioact. Mater. 2022, 13, 300–311. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Li, S.; Li, Y. Exosomes Derived From M2 Macrophages Facilitate Osteogenesis and Reduce Adipogenesis of BMSCs. Front. Endocrinol. 2021, 12, 680328. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chang, X.; Tian, J.; Kang, L.; Wu, Y.; Liu, J.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: Release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotechnol. 2021, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl. Mater. Interfaces 2021, 13, 18472–18487. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lin, D.; Zhao, H.; Chen, L.; Cai, B.; Lin, K.; Shen, S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials 2021, 272, 120718. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, R.; Yang, Y.; Liang, C.; Yu, X.; Liu, Y.; Wang, T.; Yu, Y.; Deng, F. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J. Nanobiotechnol. 2021, 19, 78. [Google Scholar] [CrossRef]
- Fan, L.; Guan, P.; Xiao, C.; Wen, H.; Wang, Q.; Liu, C.; Luo, Y.; Ma, L.; Tan, G.; Yu, P.; et al. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact. Mater. 2021, 6, 2754–2766. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Y.; Yu, L.; Zou, L.; Yang, L.; Lin, S.; Wang, J.; Yuan, Z.; Dai, J. Exosomal miR-335 derived from mature dendritic cells enhanced mesenchymal stem cell-mediated bone regeneration of bone defects in athymic rats. Mol. Med. 2021, 27, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, F.; Li, L.; Zhou, L.; Zhou, T.; Xu, Y.; Lin, K.; Fang, B.; Xia, L. Bone marrow stromal cells stimulated by strontium-substituted calcium silicate ceramics: Release of exosomal miR-146a regulates osteogenesis and angiogenesis. Acta Biomater. 2021, 119, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control Release 2017, 251, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Su, J.; An, M.; Yang, Y.; Zhang, Y.; Zuo, J.; Zhang, N.; Zhao, Y. Novel DEK-Targeting Aptamer Delivered by a Hydrogel Microneedle Attenuates Collagen-Induced Arthritis. Mol. Pharm. 2021, 18, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.F. Parathyroid hormone (1-34)-coated microneedle patch system: Clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kelekis, A.; Filippiadis, D.K.; Kelekis, N.L.; Martin, J.B. Percutaneous augmented osteoplasty of the humeral bone using a combination of microneedles mesh and cement. J. Vasc. Interv. Radiol. 2015, 26, 595–597. [Google Scholar] [CrossRef]
- Katsumi, H.; Liu, S.; Tanaka, Y.; Hitomi, K.; Hayashi, R.; Hirai, Y.; Kusamori, K.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; et al. Development of a novel self-dissolving microneedle array of alendronate, a nitrogen-containing bisphosphonate: Evaluation of transdermal absorption, safety, and pharmacological effects after application in rats. J. Pharm. Sci. 2012, 101, 3230–3238. [Google Scholar] [CrossRef]
- Katsumi, H.; Tanaka, Y.; Hitomi, K.; Liu, S.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis. Pharmaceutics 2017, 9, 29. [Google Scholar] [CrossRef]
- Carswell, A.T.; Eastman, K.G.; Casey, A.; Hammond, M.; Shepstone, L.; Payerne, E.; Toms, A.P.; MacKay, J.W.; Swart, A.M.; Greeves, J.P.; et al. Teriparatide and stress fracture healing in young adults (RETURN—Research on Efficacy of Teriparatide Use in the Return of recruits to Normal duty): Study protocol for a randomised controlled trial. Trials 2021, 22, 580. [Google Scholar] [CrossRef]
- Cosman, F.; Lane, N.E.; Bolognese, M.A.; Zanchetta, J.R.; Garcia-Hernandez, P.A.; Sees, K.; Matriano, J.A.; Gaumer, K.; Daddona, P.E. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J. Clin. Endocrinol. Metab. 2010, 95, 151–158. [Google Scholar] [CrossRef]
- Ameri, M.; Fan, S.C.; Maa, Y.F. Parathyroid hormone PTH(1-34) formulation that enables uniform coating on a novel transdermal microprojection delivery system. Pharm. Res. 2010, 27, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Naito, C.; Katsumi, H.; Suzuki, T.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Self-Dissolving Microneedle Arrays for Transdermal Absorption Enhancement of Human Parathyroid Hormone (1-34). Pharmaceutics 2018, 10, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arlt, H.; Besschetnova, T.; Ominsky, M.S.; Fredericks, D.C.; Lanske, B. Effects of systemically administered abaloparatide, an osteoanabolic PTHrP analog, as an adjuvant therapy for spinal fusion in rats. JOR Spine 2021, 4, e1132. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Abaloparatide: First Global Approval. Drugs 2017, 77, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Mansoor, S.; Kalluri, H.; Zarnitsyn, V.G.; Choi, S.O.; Banga, A.K.; Prausnitz, M.R. Delivery of salmon calcitonin using a microneedle patch. Int. J. Pharm. 2012, 423, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Y.; Wei, F.; Liu, H.; Wang, Y.; Zhao, W.; Dong, Z.; Ma, T.; Wang, Q. Transdermal Delivery of Salmon Calcitonin Using a Dissolving Microneedle Array: Characterization, Stability, and In vivo Pharmacodynamics. AAPS PharmSciTech 2020, 22, 1. [Google Scholar] [CrossRef]
- Targeted Delivery of Anesthetic Agents to Bone Tissues using Conductive Microneedles Enhanced Iontophoresis for Painless Dental Anesthesia. Adv. Funct. Mater. 2021, 31, 2105686. [CrossRef]
- Hourdé, C.; Joanne, P.; Medja, F.; Mougenot, N.; Jacquet, A.; Mouisel, E.; Pannerec, A.; Hatem, S.; Butler-Browne, G.; Agbulut, O.; et al. Voluntary physical activity protects from susceptibility to skeletal muscle contraction-induced injury but worsens heart function in mdx mice. Am. J. Pathol. 2013, 182, 1509–1518. [Google Scholar] [CrossRef]
- Kiuru, M.J.; Pihlajamäki, H.K.; Ahovuo, J.A. Bone stress injuries. Acta Radiol. 2004, 45, 317–326. [Google Scholar] [CrossRef]
- Horn, A.; Van der Meulen, J.H.; Defour, A.; Hogarth, M.; Sreetama, S.C.; Reed, A.; Scheffer, L.; Chandel, N.S.; Jaiswal, J.K. Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci. Signal. 2017, 10, 1978. [Google Scholar] [CrossRef] [Green Version]
- Lass, A.; Sohal, B.H.; Weindruch, R.; Forster, M.J.; Sohal, R.S. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic. Biol. Med. 1998, 25, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Cerri, D.G.; Rodrigues, L.C.; Alves, V.M.; Machado, J.; Bastos, V.A.F.; Carmo, K.I.D.; Alberici, L.C.; Costa, M.C.R.; Stowell, S.R.; Cummings, R.D.; et al. Endogenous galectin-3 is required for skeletal muscle repair. Glycobiology 2021, 18, 1295–1307. [Google Scholar] [CrossRef] [PubMed]


| Bone Substitute | Company and Location | Composition | Indication | Pore or Particle Size | Incorporated | References |
|---|---|---|---|---|---|---|
| k-IBS® | AritMedical, Spain | Hydroxyapatite (HA) and β-Tricalcium Phosphate (β-TCP) The HA/-TCP ratio was 3/1 | Solitary enchondroma in the hand bones | [78] | ||
| InterOss® | Sigma, USA | Mixing bovine hydroxyapatite granules to porcine derived collagen in water in 9:1 ratio (by weight) | Fill or reconstruct periodontal and bony defects in the mouth | [79] | ||
| Bontree® | HudensBio Co., Gwangju, Korea | OCP and HA mixed at a weight ratio of 80:20 | Alveolar ridge or sinus augmentation | 0.5–1 mm | [80] | |
| CustomBone® | DePuy Synthes, USA | 60% calcium sulfate and 40% HA | Human tibial fractures | [81] | ||
| Traumacem™ V+ | DePuy Synthes, USA | Acrylic bone cement in conjunction with ceramics consisting of 45% PMMA, 40% zirconium dioxide, 14.5% hydroxyapatite, and 0.5% benzoyl peroxide | Calcaneal fracture | [82] | ||
| Vitoss BA® | Stryker, Kalamazoo, USA | β-TCP particles bonded on a collagen matrix supplemented with 20 wt% 45S5 bioactive glass particles | Metaphyseal bone defect | 90–150 μm | [83] | |
| HydroSet™ | Tetracalcium phosphate (73%), dicalcium phosphate anhydrous (27%) and Na2HPO4, NaH2PO4 and Polyvinylpyrrolidone | Bone defect, skeletal fractures, hip replacements | [84] | |||
| MIIG® X3 | Wright Medical Technolog, Inc., Arlington, TN | Calcium sulfate | Comminuted calcaneal fractures | [85] | ||
| Calciresorb C35® | Ceraver, USA | Macroporous biphasic calcium phosphate ceramic granules (HA/TCP = 65/35) | Femoral bone defect | 6 mm | Mesenchymal stem cells | [86] |
| ChronOS® | Depuy Synthes, Massachusetts, USA | TCP | Bone defect | 5.03 ± 1.90 μm | [87] | |
| Graftys® | Aix-en-Provence, France | α-tricalcium phosphate, dicalcium phosphate dihydrate, monocalcium monohydrate, calcium-deficient hydroxyapatite, hydroxypropyl methyl cellulose | Bone defect | [88] | ||
| Cerament® | 60% calcium sulfate (CS) and 40% HA | Acute traumatic depression fractures of the proximal tibia | [89] | |||
| Bio-Oss® | Geistlich, Wolhusen, Switzerland | 90% DBBM extracted from cattle and 10% highly purified porcine collagen matrix | Alveolar bone resorption | 0.25–1 mm | [90] | |
| Healos® | DePuy Orthopaedics, Inc. | Osteoconductive sponge made of collagen fibers coated with hydroxyapatite | Bone defect | Recombinant human bone morphogenetic protein-2 | [91] | |
| SINTlife® | Fin-Ceramica, SpA, Faenza, Italy | Nano-structured Mg-enriched hydroxyapatite | Bone defect | 30–40 nm | [92] | |
| DBSint® | Fin-Ceramica, SpA, Faenza, Italy | Nano-structured Mg-enriched hydroxyapatite and human demineralized bone matrix | Bone defect | [92] | ||
| OsteoSet®2 demineralised bone matrix | Wright Medical Group Inc., Memphis, Tennessee, USA | DBM particles homogenously dispersed throughout surgical-grade calcium sulphate | Large osteonecrotic lesions of the femoral head | 3.5–4.8 mm | [93] | |
| OCS-B® | Calf bone powder, bone inorganic material in calf bone | Bone defect | 0.2–1 mm | [94] | ||
| BoneSource® | Stryker Orthopaedics, Mahwah, New Jersey | An equimolar mixture of tetracalcium phosphate and anhydrous dicalcium phosphate | Bone defect | 33.4 ± 6.2 μm | [95,96] | |
| Ostim® | aap biomaterials GmbH, Dieburg, Germany | Nanosized HA and calcium sulphate | Metaphyseal osseous volume defects | 19 nm | [97,98] | |
| Cortoss™ | Orthovita®, Malvern, USA | Acrylic resin reinforced with glass ceramic particles, 30% copolymerizing organic components and 70% glass-ceramic fillers | Calvarial defects | 148.4 ± 70.6 μm | [96,99] | |
| Calcibon® | Biomet-Merck Biomaterials GmbH, Darmstadt, Germany | 61% alpha-TCP, 26% calcium-hydrogeno-phosphate, 10% calcium-carbonate and 3% hydroxyl-apatite | Acute traumatic compression vertebral fracture without neurological deficit | 41.6 ± 22.0 μm | [96,100] | |
| α-BSM® | Apatitic calcium phosphate | Articular depression fractures | 12–14 nm | [101] | ||
| Norian SRS® | Monocalcium phosphate, tricalcium phosphate, calcium carbonate and sodium phosphate | Distal radial fracture | 47.2 ± 21.9 μm | [96,102] |
| Principle | Method | Advantage | Disadvantage | Materials and Bio-ink | Application | Reference |
|---|---|---|---|---|---|---|
| Laser or high energy density heat source | Stereo lithography appearance, SLA | Fast processing speed; high maturity; high precision | High cost; software operation difficulty; high environmental requirements | Hydroxyapatite; calcium chloride and diammonium hydrogen phosphate | parietal bone; cancellous bone repair; | [147,148] |
| Selected laser sintering, SLS | Wide selection of materials; without add organic adhesives; | High cost and low efficiency; | titanium alloy; alendronate-collagen; PVA-HA | segmental bone defects; alveolar bone implant therapy; | [132,149,150] | |
| Spray forming technology | Fused deposition modeling, FDM | Low cost; simple manufacturing; wide application range; | Low precision; rough surface; slow speed | PLGA; PCL-deferoxamine | cancellous bone formation; segmental bone defect | [151,152] |
| 3D printing, 3DP | Printable active substance; prepared complex scaffolds; | Drying time is long; ink is easy to deteriorate | HA powders, air jet milling powders, spherical powder | Mandibular defect; | [153] | |
| Direct ink writing 3D printing (DIW) | fast printing speed; easy operation; low cost; high precision; | Low molding accuracy; easy to deform [154]. | PLA/CA | Craniomaxillofacial Reconstruction | [155] |
| Origin of Exosomes | Target | Application | References |
|---|---|---|---|
| human mesenchymal stem cells exosomes | MiR-29a | mice with nonhealing skull defects | [167] |
| Osteogenic Human exosomes | MiR-199b/MiR-218/MiR-148a/MiR-135b/MiR-221 | human bone marrow-derived mesenchymal stem cells; osteoblast cells | [168] |
| Human bone marrow stromal/stem cell exosomes | MiR-196a/MiR-27a/MiR-206 | bone formation in Sprague Dawley (SD) rats with calvarial defects; osteoblast cells | [169] |
| human-induced pluripotent stem cell-derived mesenchymal stem cells exosomes | Akt/p-Akt | human bone marrow-derived mesenchymal stem cells | [170] |
| stem cells from apical papilla-derived exosomes | MiRNA-126-5p/MiRNA-150-5p | the mandibular defects of diabetic rats | [171] |
| mesenchymal stem cells exosomes | green fluorescent protein (GFP) | old male C57BL/6 mice | [172] |
| Bone marrow mesenchymal stem cells exosomes | Smad/RUNX2 | acute rotator cuff rupture in rabbits | [173] |
| M2 macrophagy-derived exosomal | MiRNA-26a-5p | Osteogenic differentiation of BMSCs | [174] |
| Exosomes of human umbilical vein endothelial cells | Pd-1 on the surface of T cells | callus formation and fracture healing in a murine model | [175] |
| Exosomes of M2 macrophages | MiR-690 / IRS-1/TAZ | bone marrow mesenchymal stem cells | [176] |
| Exosomes of bone mesenchymal stem cells | MiR-1260a | calvarial defect rat model. | [177] |
| Exosomes derived from mesenchymal stem cells | MiR-21/NOTCH1/DLL4 | skull defects in rats. | [178] |
| Exosomes derived from mesenchymal stem cells | Acvr2b/Acvr1 | rat skull defect model | [179] |
| Exosomes derived from bone marrow mesenchymal stem cells | RAB27B/SMPD3 | Human bone marrow mesenchymal stem cells; osteogenic cells; SD rats | [180] |
| Exosomes derived from bone marrow stem cells | NF-κB | BMSC. rat balloon models and rat femoral borehole models | [181] |
| Exosomes of mature dendritic cells | large tongue suppressor kinase 1 (LATS1) | femoral bone defect in athymic rats | [182] |
| Exosomes derived from bone marrow stromal cells | MiR-146a | human umbilical vein endothelial cells; distal femur defect in rats. | [183] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. https://doi.org/10.3390/ph15070879
Xue N, Ding X, Huang R, Jiang R, Huang H, Pan X, Min W, Chen J, Duan J-A, Liu P, et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals. 2022; 15(7):879. https://doi.org/10.3390/ph15070879
Chicago/Turabian StyleXue, Nannan, Xiaofeng Ding, Rizhong Huang, Ruihan Jiang, Heyan Huang, Xin Pan, Wen Min, Jun Chen, Jin-Ao Duan, Pei Liu, and et al. 2022. "Bone Tissue Engineering in the Treatment of Bone Defects" Pharmaceuticals 15, no. 7: 879. https://doi.org/10.3390/ph15070879