Effect of Structural Differences in Naringenin, Prenylated Naringenin, and Their Derivatives on the Anti-Influenza Virus Activity and Cellular Uptake of Their Flavanones
Abstract
:1. Introduction
2. Results
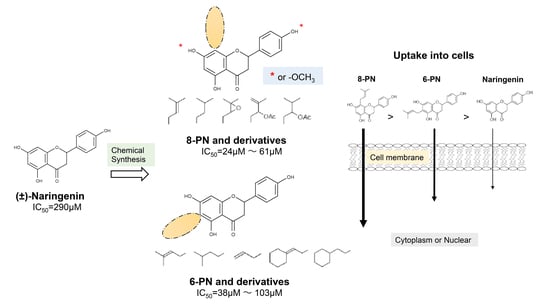
2.1. Anti-Influenza Activity of Prenylated Naringenin (8-PN, 6-PN) and Other Derivatives (1a–2d)
2.2. Coordination Effect of Modifying Groups on the Naringenin Skeleton 8-Position
2.3. Stereoscopic Effect of Modifying Groups on the Naringenin Skeleton
2.4. Uptake of Naringenin and Prenylated Naringenin into Madin–Darby Canine Kidney (MDCK) Cells
2.5. Intracellular Distribution of Naringenin and Prenylated Naringenin
2.6. Extracellular Kinetics of Prenylated Naringenin
2.7. Growth of Influenza Virus Was Inhibited by Prenylated Naringenin
3. Discussion
4. Materials and Methods
4.1. General
4.2. Cell and Viruses
4.3. Cell Viability Determination
4.4. Determination of Cell Viability in the Presence of Naringenin Derivatives
4.5. Cellular Uptake of Naringenin and Its Derivatives
4.6. Estimation of Intracellular Naringenin and Prenylated Naringenin
4.7. Time-of-Addition Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawakami, T.; Karako, K.; Song, P. Behavioral changes adopted to constrain COVID-19 in Japan: What are the implications for seasonal influenza prevention and control? Glob. Health Med. 2021, 3, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.; Lee, A.C.; Chan, J.F.; Liu, F.; Li, C.; Chen, Y.; Chu, H.; Lau, S.Y.; Wang, P.; Chan, C.C.; et al. Coinfection by severe acute respiratory syndrome coronavirus 2 and influenza A(H1N1) pdm09 virus enhances the severity of pneumonia in golden. Clin. Infect. Dis. 2021, 72, e978–e992. [Google Scholar] [CrossRef]
- Drake, T.; Ho, A.; Turtle, L.; Russell, C.; Harrison, E.; Semple, C. Influenza infection in patients hospitalized with COVID-19: Rapid Report from CO-CIN Data. In Proceedings of the 59 SAGE Meeting, London, UK, 24 September 2020. [Google Scholar]
- Honigsbaum, M. Revisiting the 1957 and 1968 influenza pandemics. Lancet 2020, 395, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Elderfield, R.; Barclay, W. Influenza pandemics. Adv. Exp. Med. Biol. 2011, 719, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Boonnak, K.; Mansanguan, C.; Schuerch, D.; Boonyuen, U.; Lerdsamran, H.; Jiamsomboon, K.; Sae Wong, F.; Huntrup, A.; Prasertsopon, J.; Kosoltanapiwat, N.; et al. Molecular characterization of seasonal influenza A and B from hospitalized patients in thailand in 2018–2019. Viruses 2021, 13, 977. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, N.; Kim, M.S.; Kang, C.; Chung, Y.S. Characterization of neuraminidase inhibitor-resistant influenza virus isolates from immunocompromised patients in the republic of korea. Virol. J. 2020, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Sugaya, N.; Hirotsu, N.; Lee, N.; de Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; et al. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N. Engl. J. Med. 2018, 379, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: A narrative review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Sina, A.A.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Santhi, V.P.; Masilamani, P.; Sriramavaratharajan, V.; Murugan, R.; Gurav, S.S.; Sarasu, V.P.; Parthiban, S.; Ayyanar, M. Therapeutic potential of phytoconstituents of edible fruits in combating emerging viral infections. J. Food Biochem. 2021, 45, e13851. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World. J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, P.; Wu, H.; Shi, R.; Su, W.; Wang, Y.; Li, P. Evaluation of naringenin as a promising treatment option for COPD based on literature review and network pharmacology. Biomolecules 2020, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef] [PubMed]
- Thayumanavan, G.; Jeyabalan, S.; Fuloria, S.; Sekar, M.; Ravi, M.; Selvaraj, L.K.; Bala, L.; Chidambaram, K.; Gan, S.H.; Rani, N.N.I.M.; et al. Silibinin and naringenin against bisphenol A-Induced neurotoxicity in zebrafish model-potential flavonoid molecules for new drug design, development, and therapy for neurological disorders. Molecules 2022, 27, 2572. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Niessner, H.; Sinnberg, T.; Berger, A.; Burkard, M.; Urmann, C.; Donaubauer, K.; Böcker, A.; Leischner, C.; Riepl, H.; et al. 6- and 8-prenylnaringenin, novel natural histone deacetylase inhibitors found in hops, exert antitumor activity on melanoma cells. Cell. Physiol. Biochem. 2018, 51, 543–556. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic perspectives of 8-prenylnaringenin, a potent phytoestrogen from hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grienke, U.; Richter, M.; Walther, E.; Hoffmann, A.; Kirchmair, J.; Makarov, V.; Nietzsche, S.; Schmidtke, M.; Rollinger, J.M. Discovery of prenylated flavonoids with dual activity against influenza virus and Streptococcus pneumoniae. Sci. Rep. 2016, 6, 27156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanada, A.; Morimoto, R.; Horio, Y.; Shichiri, M.; Nakashima, A.; Ogawa, T.; Suzuki, K.; Sumitani, H.; Ogata, T.; Isegawa, Y. Influenza virus entry and replication inhibited by 8-prenylnaringenin from Citrullus lanatus var. citroides (wild watermelon). Food Sci. Nutr. 2022, 10, 926–935. [Google Scholar] [CrossRef]
- Seliger, J.M.; Misuri, L.; Maser, E.; Hintzpeter, J. The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J. Enzyme Inhib. Med. Chem. 2018, 33, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Tronina, T.; Popłoński, J.; Bartmańska, A. Flavonoids as phytoestrogenic components of hops and beer. Molecules, 2020; 25, 4201. [Google Scholar] [CrossRef]
- Morimoto, R.; Hanada, A.; Matsubara, C.; Horio, Y.; Sumitani, H.; Ogata, T.; Isegawa, Y. Anti-influenza A virus activity of flavonoids in vitro: A structure-activity relationship. J. Nat. Med. 2022, in press. [Google Scholar] [CrossRef]
- Mukai, R.; Fujikura, Y.; Murota, K.; Uehara, M.; Minekawa, S.; Matsui, N.; Kawamura, T.; Nemoto, H.; Terao, J. Prenylation enhances quercetin uptake and reduces efflux in Caco-2 cells and enhances tissue accumulation in mice fed long-term. J. Nutr. 2013, 143, 1558–1564. [Google Scholar] [CrossRef] [Green Version]
- Wolff, H.; Motyl, M.; Hellerbrand, C.; Heilmann, J.; Kraus, B. Xanthohumol uptake and intracellular kinetics in hepatocytes, hepatic stellate cells, and intestinal cells. J. Agric. Food Chem. 2011, 59, 12893–12901. [Google Scholar] [CrossRef]
- Liu, C.H.; Jassey, A.; Hsu, H.Y.; Lin, L.T. Antiviral activities of silymarin and derivatives. Molecules 2019, 24, 1552. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Sadati, S.M.; Gheibi, N.; Ranjbar, S.; Hashemzadeh, M.S. Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidase. Biomed. Rep. 2019, 10, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zima, V.; Radilová, K.; Kožíšek, M.; Albiñana, C.B.; Karlukova, E.; Brynda, J.; Fanfrlík, J.; Flieger, M.; Hodek, J.; Weber, J.; et al. Unraveling the anti-influenza effect of flavonoids: Experimental validation of luteolin and its congeners as potent influenza endonuclease inhibitors. Eur. J. Med. Chem. 2020, 208, 112754. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Sang, H.; Liu, M.; Chen, F.; Huang, Y.; Li, L.; Liu, S.; Yang, J. Dihydromyricetin is a new inhibitor of influenza polymerase PB2 subunit and influenza-induced inflammation. Microbes. Infect. 2020, 22, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A., Jr.; Duarte Dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef] [Green Version]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. 2020, 34, 3138–3147. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Shimizu, S.; Miyamoto, S.; Izumi, T.; Obata, A.; Kikuchi, M.; Terao, J. Unique uptake and transport of isoflavone aglycones by human intestinal caco-2 cells: Comparison of isoflavonoids and flavonoids. J. Nutr. 2002, 132, 1956–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Okuyama, H.; Nishikawa, M.; Ikushiro, S.I.; Ikeda, M.; Ishima, Y.; Ukawa, Y.; Oe, K.; Teramo, J.; Mukai, R. 8-Prenylnaringenin tissue distribution and pharmacokinetics in mice and its binding to human serum albumin and cellular uptake in human embryonic kidney cells. Food Sci. Nutr. 2022, 10, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum albumin: A multifaced enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef] [PubMed]
- Bolli, A.; Marino, M.; Rimbach, G.; Fanali, G.; Fasano, M.; Ascenzi, P. Flavonoid binding to human serum albumin. Biochem. Biophys. Res. Commun. 2010, 398, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Joyner, P.M. Protein adducts and protein oxidation as molecular mechanisms of flavonoid bioactivity. Molecules 2021, 26, 5102. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Shirai, Y.; Saito, N.; Yoshida, K.; Ashida, H. Subcellular localization of flavonol aglycone in hepatocytes visualized by confocal laser scanning fluorescence microscope. Cytotechnology 2009, 59, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, Y.; Takahashi, K.; Kuno-Maekawa, M.; Sangawa, H.; Uehara, S.; Kozaki, k.; Nomura, N.; Egawa, H.; Shiraki, K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005, 49, 981–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gester, S.; Metz, P.; Zierau, O.; Vollmer, G. An efficient synthesis of the potent phytoestrogens 8-prenylnaringenin and 6-(1,1-Dimethylallyl) naringenin by europium (III)-catalyzed claisen rearrangement. Tetrahedron 2001, 57, 1015–1018. [Google Scholar] [CrossRef]
- Tischer, S.; Metz, P. Selective C-6 prenylation of flavonoids via europiumA (III)-catalyzed claisen rearrangement and cross-metathesis. Adv. Synth. Catal. 2007, 349, 147–151. [Google Scholar] [CrossRef]
- Ogata, T.; Tanaka, M.; Ishigaki, M.; Shimizu, M.; Nishiuchi, A.; Inamoto, K.; Kimachi, T. The first enantioselective total synthesis of lantalucratin C and determination of its absolute configuration. Tetrahedron 2015, 71, 6672–6680. [Google Scholar] [CrossRef]
- Su, W.; Wang, Y. PCT Int. Appl. WO 2014094370 A1 20140626. 2014. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2014094370&_cid=JP2-LAW15C-87721-1 (accessed on 5 October 2022).
- Laettig, J.; Boehl, M.; Fischer, P.; Tischer, S.; Tietboehl, C.; Menschikowski, M.; Gutzeit, H.O.; Metz, P.; Pisabarro, M.T.; Comput, J. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: Rationale for lead design. J. Comput. Aided Mol. Des. 2007, 21, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Du Nguyen, H.; Okada, T.; Kitamura, S.; Yamaoka, S.; Horaguchi, Y.; Kasanami, Y.; Sekiguchi, F.; Tsubota, M.; Yoshida, S.; Nishikawa, H.; et al. Design and synthesis of novel anti-hyperalgesic agents based on 6-prenylnaringenin as the T-type calcium channel blockers. Bioorg. Med. Chem. 2018, 26, 4410–4427. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.; Yoshioka, K.; Nakayama, M.; Nagai, E.; Okuno, Y.; Nakashima, A.; Ogawa, T.; Suzuki, K.; Enomoto, T.; Isegawa, Y. Juice of citrullus lanatus var. citroides (wild watermelon) inhibits the entry and propagation of influenza viruses in vitro and in vivo. Food Sci. Nutr. 2021, 9, 544–552. [Google Scholar] [CrossRef]
- Nagai, E.; Iwai, M.; Koketsu, R.; Sogabe, R.; Morimoto, R.; Suzuki, Y.; Ohta, Y.; Okuno, Y.; Ohshima, A.; Enomoto, T.; et al. Inhibition of influenza virus replication by adlay tea. J. Sci. Food Agric. 2018, 98, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Hänsel, R.; Schulz, J. Desmethylxanthohumol: Isolierung aus hopfen und cyclisierung zu flavanonen. Arch. Pharm. 1988, 321, 37–40. [Google Scholar]
- Kumano, T.; Richard, S.B.; Noel, J.P.; Nishiyama, M.; Kuzuyama, T. Chemoenzymatic synthesis of prenylated aromatic small molecules using streptomyces prenyltransferases with relaxed substrate specificities. Bioorg. Med. Chem. 2008, 16, 8117–8126. [Google Scholar]







| Compound Name | Structure | IC50 a (μM) | SI b |
|---|---|---|---|
| (±)-Naringenin (Na) |  | 290 ± 16.2 | 4.9 |
| Naringenin-4′,7′-diacetate (Na-di) |  | ND | ND |
| 8-prenylnaringenin (8-PN) |  | 24 ± 0.6 | 4.9 |
| 6-prenylnaringenin (6-PN) |  | 38 ± 4.7 | 5.5 |
| 1a |  | 59 ± 9.0 | 2.7 |
| 1b |  | ND | ND |
| 1e |  | ND | ND |
| 1c |  | 42 ± 2.1 | 6.3 |
| 1d |  | 61 ± 14.0 | 3.0 |
| 2a |  | 103 ± 3.8 | 0.8 |
| 2b |  | 58 ± 15.2 | 1.3 |
| 2c |  | ND | ND |
| 2d |  | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, R.; Matsubara, C.; Hanada, A.; Omoe, Y.; Ogata, T.; Isegawa, Y. Effect of Structural Differences in Naringenin, Prenylated Naringenin, and Their Derivatives on the Anti-Influenza Virus Activity and Cellular Uptake of Their Flavanones. Pharmaceuticals 2022, 15, 1480. https://doi.org/10.3390/ph15121480
Morimoto R, Matsubara C, Hanada A, Omoe Y, Ogata T, Isegawa Y. Effect of Structural Differences in Naringenin, Prenylated Naringenin, and Their Derivatives on the Anti-Influenza Virus Activity and Cellular Uptake of Their Flavanones. Pharmaceuticals. 2022; 15(12):1480. https://doi.org/10.3390/ph15121480
Chicago/Turabian StyleMorimoto, Ryosuke, Chiaki Matsubara, Akari Hanada, Yuta Omoe, Tokutaro Ogata, and Yuji Isegawa. 2022. "Effect of Structural Differences in Naringenin, Prenylated Naringenin, and Their Derivatives on the Anti-Influenza Virus Activity and Cellular Uptake of Their Flavanones" Pharmaceuticals 15, no. 12: 1480. https://doi.org/10.3390/ph15121480