Novel Aporphine- and Proaporphine–Clerodane Hybrids Identified from the Barks of Taiwanese Polyalthia longifolia (Sonn.) Thwaites var. pendula with Strong Anti-DENV2 Activity
Abstract
:1. Introduction
2. Results
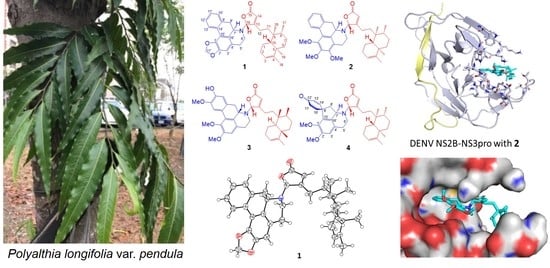
2.1. Structure Elucidation of Polyalongarins A–D (1–4)
2.2. Anti-DENV2 Activities of Polyalongarins A–D (1–4)
2.3. Molecular Docking Analysis of Polyalongarins and DENV2 NS2B-NS3pro
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Spectral Measurements
4.5. X-ray Crystallographic Data of Polyalongarin A (1)
4.6. Cytotoxic Assay
4.7. In Vitro Anti-DENV2 Assay
4.8. Western Blotting Analysis
4.9. In Silico Molecular Docking by PyRx
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Vannice, K.S.; Wilder-Smith, A.; Barrett, A.D.T.; Carrijo, K.; Cavaleri, M.; Silva, A.; Durbin, A.P.; Endy, T.; Harris, E.; Innis, B.L.; et al. Clinical development and regulatory points for consideration for second-generation live attenuated dengue vaccines. Vaccine 2018, 36, 3411–3417. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Ghosh, S.; Bell, J.A.; Sherman, W.; Hardy, J.A. Allosteric Inhibition of the NS2B-NS3 Protease from Dengue Virus. ACS Chem. Biol. 2013, 8, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huo, T.; Lin, Y.L.; Nie, S.; Wu, F.; Hua, Y.; Wu, J.; Kneubehl, A.R.; Vogt, M.B.; Rico-Hesse, R.; et al. Discovery, X-ray crystallography and antiviral activity of allostericinhibitors of flavivirus NS2B-NS3 protease. J. Am. Chem. Soc. 2019, 141, 6832–6836. [Google Scholar] [CrossRef]
- Erbel, P.; Schiering, N.; D’Arcy, A.; Renatus, M.; Kroemer, M.; Lim, S.P.; Yin, Z.; Keller, T.H.; Vasudevan, S.G.; Hommel, U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 2006, 13, 372–373. [Google Scholar] [CrossRef]
- Tietze, L.F.; Bell, H.P.; Chandrasekhar, S. Natural product hybrids as new leads for drug discovery. Angew. Chem. Int. Ed. 2003, 42, 3996–4028. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; Bolzani, V.d.S.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Liu, J.; Liu, A.; Hu, Y. Enzymatic dimerization in the biosynthetic pathway of microbial natural products. Nat. Prod. Rep. 2021, 38, 1469–1505. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Singh, S.P.; Kant, R.; Maulik, P.R.; Sarkar, J.; Kanojiya, S.; Kumar, K.R. Cytotoxic cycloartane triterpene and rare isomeric bisclerodane diterpenes from the leaves of Polyalthia longifolia var. pendula. Bioorg. Med. Chem. Lett. 2010, 20, 5767–5771. [Google Scholar] [CrossRef]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Yodbuddee, D.; Phonkerd, N. New antimalarial bis-dehydroaporphine alkaloids from Polyalthia debilis. J. Nat. Prod. 2003, 66, 616–619. [Google Scholar] [CrossRef]
- Huan, T.C.; Shieh, W.C.; Keng, H.; Tsai, J.L.; Hsieh, C.F. Flora of Taiwan, 2nd ed.; Department of Botany, National Taiwan University: Taipei, Taiwan, 1993. [Google Scholar]
- Misra, P.; Sashidhara, K.V.; Singh, S.P.; Kumar, A.; Gupta, R.; Chaudhaery, S.S.; Gupta, S.S.; Majumder, H.K.; Saxena, A.K.; Dube, A. 16α-Hydroxycleroda-3,13(14)Z-dien-15,16-olide from Polyalthia longifolia: A safe and orally active antileishmanial agent. Br. J. Pharmacol. 2016, 159, 1143–1150. [Google Scholar] [CrossRef]
- Pu, D.B.; Zhang, X.J.; Bi, D.W.; Gao, J.B.; Yang, Y.; Li, X.L.; Lin, J.; Li, X.N.; Zhang, R.H.; Xiao, W.L. Callicarpins, two classes of rearranged ent-clerodane diterpenoids from callicarpa plants blocking NLRP3 inflammasome-induced pyroptosis. J. Nat. Prod. 2020, 83, 2191–2199. [Google Scholar] [CrossRef]
- Chang, F.R.; Hwang, T.L.; Yang, Y.L.; Li, C.E.; Wu, C.C.; Issa, H.H.; Hsieh, W.B.; Wu, Y.C. Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. pendula. Planta Med. 2006, 72, 1344–1347. [Google Scholar] [CrossRef]
- Annan, K.; Ekuadzi, E.; Asare, C.; Sarpong, K.; Pistorius, D.; Oberer, L.; Gyan, B.A.; Ofori, M. Antiplasmodial constituents from the stem bark of Polyalthia longifolia var pendula. Phytochem. Lett. 2015, 11, 28–31. [Google Scholar] [CrossRef]
- Gbedema, S.Y.; Bayor, M.T.; Annan, K.; Wright, C.W. Clerodane diterpenes from Polyalthia longifolia (Sonn) Thw. var. pendula: Potential antimalarial agents for drug resistant Plasmodium falciparum infection. J. Ethnopharmacol. 2015, 169, 176–182. [Google Scholar] [CrossRef]
- Chanda, S.; Nair, R. Antimicrobial activity of Polyalthia longifolia (Sonn.) Thw. var. pendula leaf extracts against 91 clinically important pathogenic microbial strains. Chin. Med. 2010, 1, 31–38. [Google Scholar] [CrossRef]
- Khan, A.K.; Ahmed, A.; Hussain, M.; Khan, I.A.; Ali, S.A.; Farooq, A.D.; Faizi, S. Antibiofilm potential of 16-oxo-cleroda-3,13(14)E-diene-15 oic acid and its five new γ-amino γ-lactone derivatives against methicillin resistant Staphylococcus aureus and Streptococcus mutans. Eur. J. Med. Chem. 2017, 138, 480–490. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef]
- Bhakuni, D.S.; Tewari, S.; Dhar, M.M. Aporphine alkaloids of annona squamosa. Phytochemistry 1972, 11, 1819–1822. [Google Scholar] [CrossRef]
- Lightner, D.A.; Gurst, J.E. Organic Conformational Analysis and Stereochemistry from Circular Dichroism Spectroscopy; John Wiley and Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Kerwin, S.M. ChemBioOffice Ultra 2010 Suite. J. Am. Chem. Soc. 2010, 132, 2466–2467. [Google Scholar] [CrossRef]
- Flor, S.C.; Doorenbos, N.J.; Svoboda, G.H.; Knapp, J.E.; Schiff, P.L. Chemical constituents of Legnephora moorei Miers (Menispermaceae). J. Pharm. Sci. 1974, 63, 618–619. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Tseng, C.K.; Lin, C.K.; Hsu, Y.C.; Wu, Y.H.; Hsieh, C.L.; Lee, J.C. Celastrol inhibits dengue virus replication via up-regulating type I interferon and downstream interferon-stimulated responses. Antivir. Res. 2017, 137, 49–57. [Google Scholar] [CrossRef]
- Yang, C.C.; Hu, H.S.; Wu, R.H.; Wu, S.H.; Lee, S.J.; Jiaang, W.T.; Chern, J.H.; Huang, Z.S.; Wu, H.N.; Chan, C.M.; et al. A novel Dengue virus inhibitor, BP13944, discovered by high-throughput screening with dengue virus replicon cells selects for resistance in the viral NS2B/NS3 protease. Antimicrob. Agents Chemother. 2014, 58, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Hewage, R.T.; Huang, R.J.; Lai, S.J.; Lien, Y.C.; Weng, S.H.; Li, D.H.; Chen, Y.J.; Wu, S.H.; Chein, R.J.; Lin, H.C. An enzyme-mediated aza-michael addition is involved in the biosynthesis of an imidazoyl hybrid product of conidiogenone B. Org. Lett. 2021, 23, 1904–1909. [Google Scholar] [CrossRef]
- Hirata, K.; Poeaknapo, C.; Schmidt JZenk, M.H. 1,2-Dehydroreticuline synthase, the branch point enzyme opening the morphinan biosynthetic pathway. Phytochemistry 2004, 65, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- De-Eknamkul, W.; Zenk, M.H. Purification and properties of 1,2-dehydroreticuline reductase from Papaver somniferum seedlings. Phytochemistry 1992, 31, 813–821. [Google Scholar] [CrossRef]
- Peters, R.J. Two rings in them all: The labdane-related diterpenoids. Nat. Prod. Rep. 2010, 27, 1521–1530. [Google Scholar] [CrossRef]
- Timiri, A.K.; Sinha, B.N.; Jayaprakash, V. Progress and prospects on DENV protease inhibitors. Eur. J. Med. Chem. 2016, 117, 125–143. [Google Scholar] [CrossRef]
- Falgout, B.; Pethel, M.; Zhang, Y.M.; Lai, C.J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991, 65, 2467–2475. [Google Scholar] [CrossRef]
- Takagi, Y.; Matsui, K.; Nobori, H.; Maeda, H.; Sato, A.; Kurosu, T.; Orba, Y.; Sawa, H.; Hattori, K.; Higashino, K.; et al. Discovery of novel cyclic peptide inhibitors of dengue virus NS2B-NS3 protease with antiviral activity. Bioorg. Med. Chem. Lett. 2017, 27, 3586–3590. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Potter, K.C.; Zi, J.C.; Hong, Y.J.; Schulte, S.; Malchow, B.; Tantillo, D.J.; Peters, R.J. Blocking Deprotonation with Retention of Aromaticity in a Plant ent-Copalyl Diphosphate Synthase Leads to Product Rearrangement. Angew. Chem. Int. Ed. 2016, 55, 634–638. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.P.; Li, L.; Li, J.J.; Xu, L.M.; Feng, J.; Liu, Y.L. Digital Gene Expression Analysis Provides Insight into the Transcript Profile of the Genes Involved in Aporphine Alkaloid Biosynthesis in Lotus (Nelumbo nucifera). Front. Plant Sci. 2017, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- The PyMOL Molecular Graphics System, Version 2.0; Schrödinger, LLC: New York, NY, USA, 2015; Available online: https://pymol.org/2/ (accessed on 15 August 2022).






| No | Polyalongarin A (1) | Polyalongarin B (2) | Polyalongarin C (3) | Polyalongarin D (4) | ||||
|---|---|---|---|---|---|---|---|---|
| 1H (mult, Hz) | 13C (mult.) | 1H (mult, Hz) | 13C (mult.) | 1H (mult, Hz) | 13C (mult.) | 1H (mult, Hz) | 13C (mult.) | |
| 1 | 1.52 (m, 2H) | 18.4 (CH2) | 1.54 (m, 2H) | 18.4 (CH2) | 1.52 (m, 2H) | 18.2 (CH2) | 1.48 (m, 2H) | 18.3 (CH2) |
| 2 | 1.98 (m) 2.10 (m) | 26.9 (CH2) | 2.00 (m) 2.10 (m) | 26.9 (CH2) | 1.95 (m, 2H) | 26.7 (CH2) | 1.86 (m) 2.03 (m) | 26.9 (CH2) |
| 3 | 5.18 (brs) | 120.2 (CH) | 5.17 (brs) | 120.2 (CH) | 5.14 (brs) | 120.3 (CH) | 5.16 (brs) | 120.1 (CH) |
| 4 | 144.4 (C) | 144.4 (C) | 144.3 (C) | 144.6 (C) | ||||
| 5 | 38.1 (C) | 38.1 (C) | 38.1 (C) | 38.2 (C) | ||||
| 6 | 1.20 (m) 1.73 (m) | 36.6 (CH2) | 1.20 (m) 1.73 (m) | 36.5 (CH2) | 1.19 (m) 1.72 (m) | 36.6 (CH2) | 1.14 (m) 1.71 (m) | 36.6 (CH2) |
| 7 | 1.37 (m) 1.44 (m) | 27.3 (CH2) | 1.45 (m, 2H) | 27.3 (CH2) | 1.42 (m) 1.44 (m) | 27.3 (CH2) | 1.42 (m, 2H) | 27.2 (CH2) |
| 8 | 1.45 (m) | 36.4 (CH) | 1.47 (m) | 36.4 (CH) | 1.50 (m) | 36.4 (CH) | 1.40 (m) | 36.4 (CH) |
| 9 | 38.7 (C) | 38.6 (C) | 38.7 (C) | 38.6 (C) | ||||
| 10 | 1.36 (dd, 10.5, 2.0) | 46.5 (CH) | 1.36 (dd, 10.5, 2.0) | 46.6 (CH) | 1.32 (m) | 46.4 (CH) | 1.31 (m) | 46.4 (CH) |
| 11 | 1.62 (m) 1.70 (m) | 35.0 (CH2) | 1.63 (m) | 34.8 (CH2) | 1.53 (m) 1.79 (m) | 35.2 (CH2) | 1.58 (m) 1.68 (m) | 34.7 (CH2) |
| 1.67 (m) | ||||||||
| 12 | 2.25 (m, 2H) | 22.2 (CH2) | 2.23 (m, 2H) | 22.1 (CH2) | 2.12 (m) 2.30 (m) | 22.0 (CH2) | 2.05 (m, 2H) | 21.9 (CH2) |
| 13 | 169.8 (C) | 169.8 (C) | 169.9 (C) | 169.0 (C) | ||||
| 14 | 5.98 (s) | 118.7 (CH) | 5.98 (s) | 118.5 (CH) | 5.98 (s) | 118.7 (CH) | 5.98 (s) | 118.6 (CH) |
| 15 | 171.5 (C) | 171.5 (C) | 171.5 (C) | 171.4 (C) | ||||
| 16 | 6.19 (s) | 93.9 (CH) | 6.17 (s) | 93.9 (CH) | 6.17 (s) | 93.8 (CH) | 5.64 (s) | 95.2 (CH) |
| 17 | 0.79 (d, 4.5) | 16.0 (CH3) | 0.79 (d, 6.0) | 16.0 (CH3) | 0.78 (d, 5.5) | 15.9 (CH3) | 0.75 (d, 5.5) | 15.9 (CH3) |
| 18 | 1.58 (s) | 19.9 (CH3) | 1.57 (s) | 19.9 (CH3) | 1.57 (s) | 19.9 (CH3) | 1.57 (s) | 19.9 (CH3) |
| 19 | 1.01 (s) | 18.0 (CH3) | 1.01 (s) | 17.9 (CH3) | 1.00 (s) | 18.0 (CH3) | 1.00 (s) | 18.0 (CH3) |
| 20 | 0.78 (s) | 18.3 (CH3) | 0.79 (s) | 18.2 (CH3) | 0.79 (s) | 18.2 (CH3) | 0.77 (s) | 18.2 (CH3) |
| 1’ | 142.8 (C) | 150.2 (C) | 145.0 (C) | 144.6 (C) | ||||
| 2’ | 146.9 (C) | 145.4 (C) | 152.2 (C) | 153.6 (C) | ||||
| 3’ | 6.56 (s) | 107.5 (CH) | 150.2 (C) | 6.58 (s) | 110.4 (CH) | 6.65 (s) | 111.8 (CH) | |
| 4’ | 126.6 (C) | 131.0 (C) | 127.4 (C) | 127.4 (C) | ||||
| 5’ | 2.63 (m) 2.94 (m) | 29.4 (CH2) | 2.75 (br d, 14.0) 2.80 (m) | 24.0 (CH2) | 2.74 (m) 2.98 (m) | 29.4 (CH2) | 2.80 (m, 2H) | 27.3 (CH2) |
| 6’ | 2.72 (m) 2.81 (m) | 41.4 (CH2) | 2.63 (tq, 11.5, 3.5) 2.83 (m) | 40.9 (CH2) | 2.70 (m) 2.80 (m) | 41.2 (CH2) | 2.81 (m, 2H) | 42.4 (CH2) |
| 7’ | 4.20 (dd, 10.5, 3.5) | 56.3 (CH) | 4.06 (br d, 14.0) | 56.8 (CH) | 4.10 (br d, 14.0) | 56.7 (CH) | 4.51 (dd, 9.0, 6.5) | 59.9 (CH) |
| 8’ | 2.83 (dd, 9.0, 3.5) | 33.7 (CH2) | 2.73 (br d, 11.0) | 33.8 (CH2) | 2.68 (m) | 33.3 (CH2) | 2.30 (dd, 11.0, 9.0) | 46.8 (CH2) |
| 3.33 (dd, 10.5, 9.0) | 3.24 (dd, 13.5, 4.0) | 3.14 (br d, 11.0) | 2.55 (dd, 11.0, 6.5) | |||||
| 9’ | 134.0 (C) | 134.4 (C) | 125.9 (C) | 6.90 (d, 10.0) | 152.8 (CH) | |||
| 10’ | 7.25 (m) | 128.4 (CH) | 7.24 (d, 7.5) | 128.1 (CH) | 6.81 (s) | 114.3 (CH) | 6.44 (d, 10.0) | 128.6 (CH) |
| 11’ | 7.25 (m) | 127.8 (CH) | 7.22 (dd, 8.0, 7.5) | 127.2 (CH) | 146.0 (C) | 186.0 (C) | ||
| 12’ | 7.34 (m) | 127.4 (CH) | 7.33 (dd, 8.0, 7.5) | 127.3 (CH) | 145.8 (C) | 6.33 (d, 10.0) | 127.8 (CH) | |
| 13’ | 8.08 (d, 8.0) | 126.9 (CH) | 8.24 (d, 7.5) | 127.8 (CH) | 8.05 (s) | 111.2 (CH) | 7.04 (d, 10.0) | 149.4 (CH) |
| 14’ | 130.9 (C) | 131.7 (C) | 123.8 (C) | 51.1 (C) | ||||
| 15’ | 116.6 (C) | 122.9 (C) | 128.6 (C) | 132.8 (C) | ||||
| 16’ | 126.5 (C) | 122.8 (C) | 117.1 (C) | 134.1 (C) | ||||
| 17’ | 5.96 (d, 1.4) | 100.9 (CH2) | ||||||
| 6.11 (d, 1.4) | ||||||||
| 1’-OMe | 3.95 (s) | 60.9 (CH3) | 3.66 (s) | 60.2 (CH3) | 3.62 (s) | 56.4 (CH3) | ||
| 2’-OMe | 3.70 (s) | 60.4 (CH3) | 3.89 (s) | 55.8 (CH3) | 3.82 (s) | 61.1 (CH3) | ||
| 3’-OMe | 3.92 (s) | 60.6 (CH3) | ||||||
| 12’-OMe | 3.92 (s) | 56.0 (CH3) | ||||||
| Compounds | IC50 (μM) a | CC50 (μM) b | SI c |
|---|---|---|---|
| polyalongarin A (1) | 4.46 ± 0.12 | >200 | 44.8 |
| polyalongarin B (2) | 2.80 ± 0.23 | 50.44 ± 10.20 | 18 |
| polyalongarin C (3) | 3.24 ± 0.16 | 69.11 ± 13.97 | 21.3 |
| polyalongarin D (4) | 6.36 ± 0.09 | 94.47 ± 10.16 | 14.9 |
| Celastrol d | 0.12 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, I.-W.; Liao, G.-Y.; Lee, J.-C.; Chang, C.-I.; Wu, Y.-C.; Chen, Y.-Y.; Liu, S.-P.; Su, H.-J.; Liu, C.-I.; Kuo, C.-Y.; et al. Novel Aporphine- and Proaporphine–Clerodane Hybrids Identified from the Barks of Taiwanese Polyalthia longifolia (Sonn.) Thwaites var. pendula with Strong Anti-DENV2 Activity. Pharmaceuticals 2022, 15, 1218. https://doi.org/10.3390/ph15101218
Lo I-W, Liao G-Y, Lee J-C, Chang C-I, Wu Y-C, Chen Y-Y, Liu S-P, Su H-J, Liu C-I, Kuo C-Y, et al. Novel Aporphine- and Proaporphine–Clerodane Hybrids Identified from the Barks of Taiwanese Polyalthia longifolia (Sonn.) Thwaites var. pendula with Strong Anti-DENV2 Activity. Pharmaceuticals. 2022; 15(10):1218. https://doi.org/10.3390/ph15101218
Chicago/Turabian StyleLo, I-Wen, Geng-You Liao, Jin-Ching Lee, Chi-I Chang, Yang-Chang Wu, Yen-Yu Chen, Shang-Pin Liu, Huey-Jen Su, Chih-I Liu, Chia-Yi Kuo, and et al. 2022. "Novel Aporphine- and Proaporphine–Clerodane Hybrids Identified from the Barks of Taiwanese Polyalthia longifolia (Sonn.) Thwaites var. pendula with Strong Anti-DENV2 Activity" Pharmaceuticals 15, no. 10: 1218. https://doi.org/10.3390/ph15101218