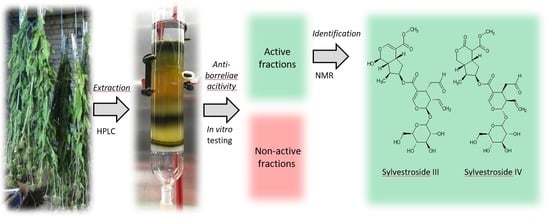
Extraction and Fractionation of Bioactives from Dipsacus fullonum L. Leaves and Evaluation of Their Anti-Borrelia Activity
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of D. fullonum Extract
3.3. Isolation of D. fullonum L. Constituents
3.4. NMR Analysis
3.5. HPLC–DAD–MS Analysis
3.6. ORAC Assay
3.7. Bacterial Strain, Media and Culture
3.8. SYBR Green I/PI Assay
3.9. Evaluation of Bactericidal Effect of Test Compounds
3.10. Cell Culture
3.11. Treatment Procedure and Sample Preparation
3.12. Cell Viability Measured by WST-1
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimering, J.H.; Williams, M.R.; Eiras, M.E.; Fallon, B.A.; Logigian, E.L.; Dworkin, R.H. Acute and chronic pain associated with Lyme borreliosis: Clinical characteristics and pathophysiologic mechanisms. Pain 2014, 155, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; D’Agosto, G.; Pontone, M.; Trento, E.; Gallo, M.T.; Prignano, G.; Pimpinelli, F.; Toma, L.; et al. The emerging role of microbial biofilm in lyme neuroborreliosis. Front. Neurol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Caskey, J.R.; Embers, M.E. Persister Development by Borrelia burgdorferi populations in vitro. Antimicrob. Agents Chemother. 2015, 59, 6288–6295. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Brown, A.V.; Matluck, N.E.; Hu, L.T.; Lewis, K. Borrelia burgdorferi, the causative agent of lyme disease, forms drug-tolerant persister cells. Antimicrob. Agents Chemother. 2015, 59, 4616–4624. [Google Scholar] [CrossRef] [Green Version]
- Stricker, R.B.; Sapi, E.; Kaur, N.; Anyanwu, S.; Luecke, D.F.; Datar, A.; Patel, S.; Rossi, M. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect. Drug Resist. 2011, 4, 97–113. [Google Scholar] [CrossRef] [Green Version]
- Aucott, J.N.; Crowder, L.; Kortte, K.B. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int. J. Infect. Dis. 2013, 17, e443–e449. [Google Scholar] [CrossRef] [Green Version]
- Brorson, Ø.; Brorson, S.-H. Grapefruit seed extract is a powerful in vitro agent against motile and cystic forms of Borrelia burgdorferi sensu lato. Infection 2007, 35, 206–208. [Google Scholar] [CrossRef]
- Theophilus, P.A.S.; Victoria, M.J.; Socarras, K.M.; Filush, K.R.; Gupta, K.; Luecke, D.F.; Sapi, E. Effectiveness of Stevia rebaudiana whole leaf extract against the various morphological forms of Borrelia burgdorferi in vitro. Eur. J. Microbiol. Immunol. 2015, 5, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Goc, A.; Niedzwiecki, A.; Rath, M. Cooperation of doxycycline with phytochemicals and micronutrients against active and persistent forms of Borrelia sp. Int. J. Biol. Sci. 2016, 12, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Goc, A.; Rath, M. The anti-borreliae efficacy of phytochemicals and micronutrients: An update. Ther. Adv. Infect. Dis. 2016, 3, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.-M.; Shi, Y.-P. Phytochemicals and biological activities of dipsacus species. Chem. Biodivers. 2011, 8, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.M.; Lee, A.Y.; Nam, J.Y.; Lim, K.S.; Choe, M.S.; Lee, M.Y.; Kim, C.; Kim, J.-S. Effects of dipsacus asperoides extract on monosodium iodoacetate–Induced osteoarthritis in rats based on gene expression profiling. Front. Pharmacol. 2021, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Rauwald, H.W.; Liebold, T.; Straubinger, R.K. Growth inhibiting activity of lipophilic extracts from Dipsacus sylvestris Huds. roots against Borrelia burgdorferi s. s. in vitro. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 628–630. [Google Scholar] [CrossRef]
- Feng, J.; Leone, J.; Schweig, S.; Zhang, Y. Evaluation of natural and botanical medicines for activity against growing and non-growing forms of B. burgdorferi. Front. Med. 2020, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oszmiański, J.; Wojdyło, A.; Juszczyk, P.; Nowicka, P. Roots and leaf extracts of Dipsacus fullonum L. and Their biological activities. Plants 2020, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Feng, X.; Xu, J.; Lei, H.; Zhang, L. Multi-component HPLC analysis and antioxidant activity characterization of extracts from Dipsacus sativus (Linn.) Honck. Int. J. Food Prop. 2016, 19, 1000–1006. [Google Scholar] [CrossRef]
- Kuhtinskaja, M.; Bragina, O.; Kulp, M.; Vaher, M. Anticancer Effect of the Iridoid Glycoside Fraction from Dipsacus fullonum L. Leaves. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Vaher, M.; Kuhtinskaja, M. Extraction and analysis of bioactive compounds from dipsacus fullonum and galium verum for lyme borreliosis treatment. Biomed. J. Sci. Tech. Res. 2018, 11, 8614–8616. [Google Scholar] [CrossRef] [Green Version]
- Sherma, J.; Fried, B. Handbook of Thin-Layer Chromatography, 3rd ed.; Marcel Dekker, Inc.: Basel, Switzerland, 2003; Volume 89. [Google Scholar]
- Ji, D.; Zhang, C.; Li, J.; Yang, H.; Shen, J.; Yang, Z. A new iridoid glycoside from the roots of dipsacus asper. Molecules 2012, 17, 1419–1424. [Google Scholar] [CrossRef]
- Zhang, H.; Rothwangl, K.; Mesecar, A.D.; Sabahi, A.; Rong, L.; Fong, H.H.S. Lamiridosins, hepatitis C virus entry inhibitors from lamium album. J. Nat. Prod. 2009, 72, 2158–2162. [Google Scholar] [CrossRef]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Min, S.-Y.; Park, C.-H.; Yu, H.-W.; Park, Y.-J. Anti-inflammatory and anti-allergic effects of saponarin and its impact on signaling pathways of RAW 264.7, RBL-2H3, and HaCaT Cells. Int. J. Mol. Sci. 2021, 22, 8431. [Google Scholar] [CrossRef]
- Jiang, Y.; Kusama, K.; Satoh, K.; Takayama, F.; Watanabe, S.; Sakagami, H. Induction of cytotoxicity by chlorogenic acid in human oral tumor cell lines. Phytomedicine 2000, 7, 483–491. [Google Scholar] [CrossRef]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug combinations against Borrelia burgdorferi Persisters in vitro: Eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS ONE 2015, 10, e0117207. [Google Scholar] [CrossRef] [Green Version]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Fujimoto, A.; Sakanashi, Y.; Matsui, H.; Oyama, T.; Nishimura, Y.; Masuda, T.; Oyama, Y. Cytometric analysis of cytotoxicity of polyphenols and related phenolics to rat thymocytes: Potent cytotoxicity of resveratrol to normal cells. Basic Clin. Pharmacol. Toxicol. 2009, 104, 455–462. [Google Scholar] [CrossRef]
- Kocsis, Á.; Szabó, L.F.; Podányi, B. New bis-iridoids from dipsacus laciniatus. J. Nat. Prod. 1993, 56, 1486–1499. [Google Scholar] [CrossRef]
- Jensen, S.R.; Lyse-Petersen, S.E.; Nielsen, B.J. Novel bis-iridoid glucosides from Dipsacus sylvestris. Phytochemistry 1979, 18, 273–277. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Guo, F.; Wu, Y.; Li, Y. Antinociceptive and anti-inflammatory activities of a standardizedextract of bis-iridoids from Pterocephalus hookeri. J. Ethnopharmacol. 2018, 216, 233–238. [Google Scholar] [CrossRef]
- Rahman, A.U.; Ahmad, V.U. 13C-NMR of Natural Products; Springer: Boston, MA, USA, 1992; Volume 1. [Google Scholar] [CrossRef]
- Naguib, Y.M. A Fluorometric Method for Measurement of Oxygen Radical-Scavenging Activity of Water-Soluble Antioxidants. Anal. Biochem. 2000, 284, 93–98. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg. Microbes Infect. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef]



| Peak | Compound | Tret, Min | λmax, nm | MS, m/z | MS/MS, m/z | Conc., mg/L |
|---|---|---|---|---|---|---|
| 1 | Chlorogenic acid | 4.7 | 245; 325 | 353 | 191 | 47.8 ± 2.7 |
| 2 | Loganic acid | 5.8 | 230 | 375 | 213; 169 | 14.6 ± 2.1 |
| 3 | Luteolin derivative | 9.3 | 270; 350 | 609 | 447; 325 | 0.8 ± 0.3 |
| 4 | Loganin | 9.5 | 238 | 389 | 227; 209 | 13.6 ± 0.7 |
| 5 | Loganic acid ethyl ester | 9.8 | 245 | 403 | 395; 357 | 6.0 ± 0.5 |
| 6 | Saponarin | 10.4 | 270; 335 | 593 | 311; 431; 473 | 48.1 ± 6.1 |
| 7 | Isoorientin | 10.7 | 347 | 447 | 557; 327; 429 | 16.3 ± 2.1 |
| 8 | Dichlorogenic acid isomer | 11.1 | 325 | 515 | 353; 191 | 39.7 ± 1.9 |
| 9 | Dichlorogenic acid isomer | 11.6 | 325 | 515 | 353; 173; 203 | 3.6 ± 3.2 |
| 10 | Isovitexin | 12.0 | 268; 340 | 431 | 311; 341; 413 | 11.7 ± 0.5 |
| 11 | bis-Iridoid glycoside | 12.9 | 238 | 541 | – | 4.4 ± 0.8 |
| 12 | bis-Iridoid glycoside | 13.5 | 240 | 585 | 373 | 0.8 ± 0.3 |
| 13 | bis-Iridoid glycoside | 13.8 | 240 | 583 | 513; 459 | 1.0 ± 0.6 |
| 14 | bis-Iridoid glycoside | 14.1 | 240 | 585 | 373 | 5.0 ± 0.7 |
| 15 | bis-Iridoid glycoside | 14.7 | 240 | 585 | 373 | 4.8 ± 0.9 |
| 16 | bis-Iridoid glycoside | 15.1 | 240 | 585 | 373 | 2.9 ± 0.4 |
| 17 | bis-Iridoid glycoside | 15.6 | 240 | 583 | 373 | 4.9 ± 1.6 |
| 18 | Sylvestrosides III and IV | 16.2 | 240 | 583 | 373 | 42.5 ± 4.7 |
| 19 | bis-Iridoid glycoside | 16.5 | 240 | 583 | 373 | 0.9 ± 0.2 |
| 20 | bis-Iridoid glycoside | 16.8 | 240 | 583 | 373 | 10.5 ± 0.1 |
| 21 | Bicalutamide (IS) | 23.3 | 215; 270 | 429 | 255; 183 | 500 |
| NP Number | Main Constituents | Activity |
|---|---|---|
| NP1 | Chlorophylls | − |
| NP2 | bis-Iridoids (m/z 585, 583) | + |
| NP3 | Iridoids—loganin, loganic acid ethyl ester | − |
| NP4 | Loganin derivatives | − |
| NP5 | Loganic acid | + |
| NP6 | Isovitexin, saponarin, isoorientin | + |
| NP7 | Saponarin, isoorientin, 2 chlorogenic acid derivatives | + |
| NP8 | Saponarin, isoorientin, chlorogenic acid | + |
| Sample | Gross Conc., mg/L | Residual Viability, % |
|---|---|---|
| DE | 305.5 ± 24.5 | 19.8 ± 4.7 |
| NP2 | 295.4 ± 12.1 | 54.3 ± 10.4 |
| NP5 | 332.8 ± 34.7 | 29.8 ± 7.8 |
| NP7 | 340.2 ± 14.5 | 23.4 ± 15.8 |
| DM | 308.6 ± 20.2 | 40.2 ± 9.1 |
| NP2-RP | 300.2 ± 8.9 | 64.5 ± 14.9 |
| Dox., Cefo., Dap. * | 22.2; 33.4; 80.1 | 24.9 ± 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saar-Reismaa, P.; Bragina, O.; Kuhtinskaja, M.; Reile, I.; Laanet, P.-R.; Kulp, M.; Vaher, M. Extraction and Fractionation of Bioactives from Dipsacus fullonum L. Leaves and Evaluation of Their Anti-Borrelia Activity. Pharmaceuticals 2022, 15, 87. https://doi.org/10.3390/ph15010087
Saar-Reismaa P, Bragina O, Kuhtinskaja M, Reile I, Laanet P-R, Kulp M, Vaher M. Extraction and Fractionation of Bioactives from Dipsacus fullonum L. Leaves and Evaluation of Their Anti-Borrelia Activity. Pharmaceuticals. 2022; 15(1):87. https://doi.org/10.3390/ph15010087
Chicago/Turabian StyleSaar-Reismaa, Piret, Olga Bragina, Maria Kuhtinskaja, Indrek Reile, Pille-Riin Laanet, Maria Kulp, and Merike Vaher. 2022. "Extraction and Fractionation of Bioactives from Dipsacus fullonum L. Leaves and Evaluation of Their Anti-Borrelia Activity" Pharmaceuticals 15, no. 1: 87. https://doi.org/10.3390/ph15010087